Preparation and characterization of ZnO-CM
ZnO-CM have been synthesized utilizing a hydrothermal one-pot technique [25, 26].As depicted in Fig. 1A, B, S2 and S3, the electron micrographs reveal that the synthesized ZnO-CM reveals a spherical morphology, with particles uniformly distributed and a dimension of roughly 430 nm (Fig. 1F). Notably, there is no such thing as a vital distinction in morphology or particle dimension when in comparison with undoped ZnO, suggesting that the incorporation of Cu and Mn doesn’t have an effect on the structural traits of the nanospheres. TEM photos show a attribute ZnO lattice fringe with a (100) crystal spacing of 0.28 nm, indicating that Cu and Mn doping doesn’t alter the crystalline construction (Fig. 1C, D). HAADF-STEM photos and EDX elemental mapping (Fig. 1E) reveal that Zn, O, Cu, and Mn are uniformly distributed all through your entire crystal lattice of ZnO-CM, additional confirming the profitable synthesis of the ZnO-CM nanospheres.
Zeta potentials for ZnO and ZnO-CM have been measured to be -19 mV and − 4.63 mV, respectively. The numerous improve within the zeta potential of ZnO-CM, in comparison with the one doping of Cu or Mn (Fig. 2A), suggesting that elemental co-doping influences the zeta potential. ICP-MS evaluation (Desk S1) decided that the contents of Cu and Mn within the ZnO-CM nanospheres are 4.7 wt % and seven.6 wt %, respectively. To investigate the crystal construction and floor chemical environments of ZnO-CM nanospheres, XRD and XPS have been carried out. As proven in Fig. 2B and Fig. S4, the ZnO samples earlier than and after Cu/Mn doping had a typical wurtzite-type part (PDF No. 79-2205) [27]. No different attribute peaks assigned to metallic Cu, Mn or oxide phases appeared in ZnO-CM, illustrating that Cu and Mn have been doped into the ZnO lattice with out altering the ZnO part construction [28]. The XPS outcomes present that, in comparison with undoped ZnO, ZnO-CM reveals sign of Cu and Mn along with Zn and O components, additional confirming the profitable doping of Cu and Mn (Fig. 2C). For the ZnO, the XPS spectrum of Zn 2p reveals two peaks situated at 1021.68 and 1044.88 eV, belonging to the 2p1/2 and 2p3/2 of Zn2+, respectively [29]. After doping with Cu and Mn, the peaks intensities lower and shift barely to greater binding vitality, suggesting that the electron density of Zn decreases owing to the doping of Cu and Mn [30] (Fig. 2D). The high-resolution XPS spectrum of Mn 2p reveals two separated peaks at 653.28 and 641.99 eV, which will be assigned to Mn 2p3/2 and Mn 2p1/2 of Mn2+ (Fig. 2E), respectively. The Cu 2p spectrum incorporates 4 dominant peaks, with the peaks at 932.79 and 952.69 eV similar to Cu 2p3/2 and Cu 2p1/2, respectively. Different peaks at 962.38 and 944.08 eV are assigned to satellites (labeled as “Sat.”). Notably, the peaks converge at 933.12, 952.66, 934.40, and 954.40 eV, signifying the binding energies of Cu(I) and Cu(II) and indicating the coexistence of each Cu(I) and Cu(II) species on the floor of ZnO-CM (Fig. 2F) [31]. The O1s spectrum are deconvoluted into three peaks (Fig. 2G), that are fitted by constituents similar to the lattice oxygen (OL), vacant oxygen (OV), and floor oxygen (OC). The depth of vacant oxygen considerably elevated, revealing that Cu and Mn doping induced the technology of a large number of oxygen vacancies. As well as, this improve in oxygen emptiness focus was additional verified by electron paramagnetic resonance (EPR) evaluation. As proven in Fig. 1G, ZnO-CM shows an intense sign at g = 2.037, similar to the electrons trapped on the oxygen emptiness, as in contrast with the EPR sign detected for undoped ZnO [17].
Analysis of enzyme-like exercise
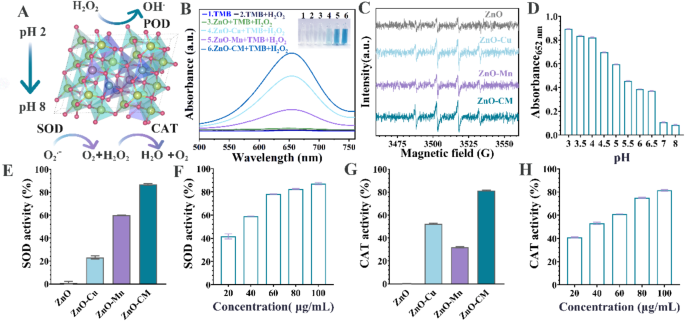
We systematically in contrast the enzymatic exercise in varied doped ZnO (Fig. 3A), particularly assessing the peroxidase-like (POD-like) exercise of ZnO-CM with 3,3’,5,5’-tetramethylbenzidine (TMB) as an indicator. TMB undergoes a colour change from colorless to blue oxTMB, demonstrating a UV-vis absorption peak at 652 nm. ZnO-CM and single-doped ZnO, particularly ZnO-Cu and ZnO-Mn, each oxidize TMB, confirming their POD-like exercise, and the catalytic exercise of ZnO-CM is considerably greater than that of the single-doped variants (Fig. 3B). Electron spin resonance (ESR) measurements have been carried out to show the technology of OH below acidic circumstances (Fig. 3C). It’s apparent that the quartet attribute DMPO-OH sign with an depth ratio of 1:2:2:1 seems in each single- and co- doped ZnO. The sign depth for ZnO-CM is considerably greater than that of ZnO doped solely with Cu or Mn, indicating that extra OH species have been produced for ZnO-CM. The pH exploration outcomes confirmed that the blue oxTMB can solely be noticed at pH = 3-6.5, whereas no colour change is noticed at pH = 7 and eight, revealing that the POD-like exercise of ZnO-CM solely occurred in acidic circumstances (Fig. 3D). Thus, ZnO-CM can generate OH for antibacterial motion within the acidic foregut whereas preserving antioxidant capabilities within the physiological hindgut (pH 7–7.8). It’s well-known that SOD is the preliminary line of resistance towards ROS in organisms as a consequence of its means to decompose O2•− [19]. We additionally evaluated the reactive oxygen species (ROS) scavenging exercise of ZnO-CM below impartial circumstances utilizing nitrotetrazolium blue chloride (NBT) [32]. ZnO-Mn and ZnO-CM exhibit elimination ratios of roughly 60% and 86.8%, respectively. ZnO-CM reveal concentration-dependent superoxide dismutase (SOD)-like exercise, reaching about 87% elimination at 100 µg mL⁻¹. Moreover, ZnO-CM exhibits wonderful catalase (CAT)-like exercise, being 1.55-fold and a pair of.56-fold greater than ZnO-Cu and ZnO-Mn, respectively, reaching roughly 81.43% H2O2 removing price on the identical focus (Fig. 3E-H).
Detection of enzyme-like activityof ZnO-CM. (A) Simulation diagram illustrating the enzyme exercise response of ZnO-CM. (B) Check outcomes for CAT enzyme exercise. (C) ESR spin resonance spectrum with a pH of three. (D) Oxidase enzyme exercise below various pH circumstances. (E) Comparability of SOD enzyme exercise at a focus of 100 µg/ml of ZnO and totally different doped ZnO variants. (F) SOD enzyme exercise check carried out below totally different concentrations of ZnO-CM. (G) CAT enzyme exercise measurements for 100 µg/ml of ZnO and varied doped ZnO samples. (H) Dedication of CAT enzyme exercise at totally different concentrations of ZnO-CM
Theoretical calculations have been carried out to elucidate the response mechanisms of SOD- and CAT-like actions in Cu/Mn single- or co-doped ZnO. The SOD-like pathway for ZnO-CM (Fig. 4A) and ZnO-Mn (Fig. S5) entails two key reactions: discount and oxidation. Within the discount part, OOH (a type of O2•− in water) [33] is absorbed to type *OOH, which captures a proton from H2O, yielding *OH-H2O2 and releasing H2O2. Within the oxidation part, the remaining *OH oxidizes a further OOH free radical to type *H2O-O2, adopted by the discharge of O2. This SOD-like cycle consists of seven states, together with two transition states (TS1/TS2); ZnO-CM reveals considerably decrease free vitality limitations (0.15 eV and 0.87 eV) than ZnO-Mn (0.75 eV and 1.65 eV) (Fig. 4B), indicating the popular reactivity of ZnO-CM. For the CAT-like pathway, H2O2 is absorbed and decomposes into H2O and the *O intermediate, which then combines with a second H2O2, producing *O2 and releasing extra water and O2 (Fig. 4C). This catalytic cycle has 5 states, together with two transition states, with ZnO-CM displaying decrease free vitality limitations (0.96 eV and 0.72 eV) in comparison with ZnO-Cu (1.53 eV and 1.10 eV) (Fig. 4D). Collectively, these findings affirm that Cu/Mn co-doping successfully enhances the antioxidant capability of ZnO, thereby enabling ZnO-CM to exhibit superior SOD-like and CAT-like exercise.
Simulation of the mechanism of ZnO-CM (A) Simulation and calculation of SOD exercise for ZnO-CM. (B) Comparability of vitality barrier adjustments between ZnO-Mn and ZnO-CM.(C) Simulation of CAT enzyme exercise for ZnO-CM and (D) Comparability of vitality barrier adjustments between ZnO-Cu and ZnO-CM reactions
Antibacterial examine in vitro
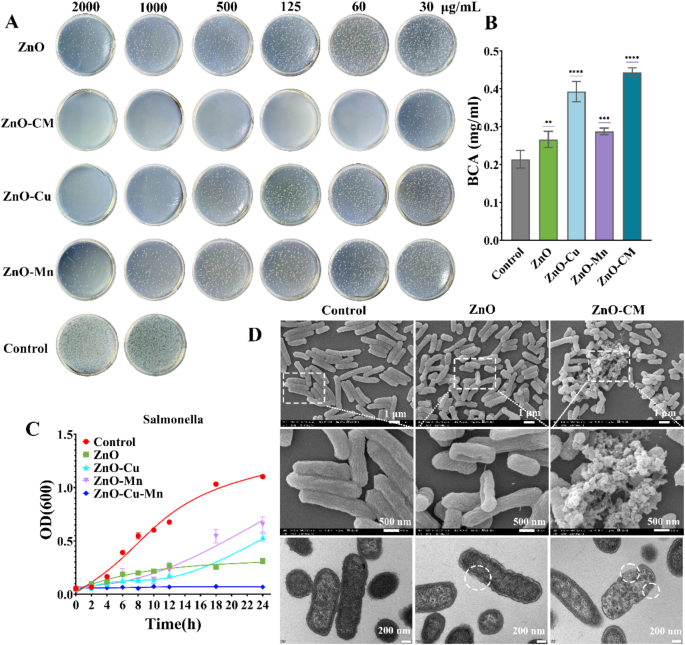
Given the enzymatic properties of ZnO-CM, we examined its antibacterial exercise in vitro. The antibacterial impact of ZnO-CM was additional decided by plate counting technique, as proven in Fig. 5A. It signifies that ZnO-CM reveals the strongest bactericidal impact, considerably lowering bacterial viability at decrease concentrations in comparison with ZnO, ZnO-Cu, and ZnO-Mn, and the inhibition price will increase with the focus of ZnO-CM. Moreover, a big discount in colony rely is noticed, however there may be nonetheless noticeable bacterial development within the 30 µg/mL remedy group. Nonetheless, the colony rely is extraordinarily low, indicating a robust bactericidal impact within the 60 µg/mL remedy group (Fig. 5A). The minimal bactericidal focus (MBC) was decided to be 125 µg/mL for ZnO-CM. At a focus of 1000 µg/mL, the antibacterial impact of ZnO-Cu is considerably enhanced, with nearly full elimination of micro organism, which can be as a result of bactericidal impact of Cu ions. ZnO doped with Mn solely demonstrates a slight bactericidal impact, and its bactericidal efficacy doesn’t considerably improve with the focus. Nonetheless, ZnO doped with each Cu and Mn reveals the very best bactericidal impact at low concentrations, reaching a bactericidal effectivity of 98% at a focus of 60 µg/mL (Fig. 5A). Cu additional enhances the bacteriostatic impact [34, 35]. Below the affect of nanomaterials, bacterial membranes might expertise injury or pore formation, after co-incubation with Salmonella for 18 h, ZnO-CM remedy considerably brought about membrane injury and structural alterations within the micro organism (Fig. 5D). When membrane injury reaches a sure threshold, it causes substantial lack of mobile contents, finally leading to bacterial loss of life. Amongst these mobile contents, proteins can function indicators [36]. Due to this fact, by using the BCA technique, we will instantly observe the amount of protein leaked into the exterior atmosphere below totally different remedies (Management, ZnO, ZnO-Cu, ZnO-Mn, ZnO-CM) on the identical dosage, the place the protein leakage measured within the ZnO-CM remedy group is 0.44 mg/L, which outperform the clean and ZnO-only teams (Fig. 5B). Moreover, the bacterial development curve reveals that at 60 µg/mL, the bacterial development within the ZnO-CM remedy group ceases over time (Fig. 5C). As a broad-spectrum antibacterial agent [37], ZnO disrupts bacterial membranes and results in protein leakage [38, 39], whereas the peroxidase exercise of ZnO-CM might produce hydroxyl radicals below sure circumstances (pH 3-6.5) to attain synergistic bactericidal impact [39].
Antibacterial results of ZnO and totally different doped ZnO in vitro. (A) MBC coating check outcomes for ZnO, ZnO-Cu, ZnO-Mn, and ZnO-CM. (B) Utilizing the BCA technique, protein leakage from micro organism was measured following 18 h of incubation with ZnO, ZnO-Cu, ZnO-Mn, and ZnO-CM at 60 µg/ml. (C) Bacterial development curves for the remedies with 60 µg/ml of ZnO, ZnO-Cu, ZnO-Mn, and ZnO-CM. (D) SEM photos and TEM photos of micro organism after 1 h of incubation with 60 µg/ml of ZnO and ZnO-CM. *Observe: Statistical significance is indicated as follows: *p < 0.05, **p < 0.01, **p < 0.001
Preliminary security evaluation
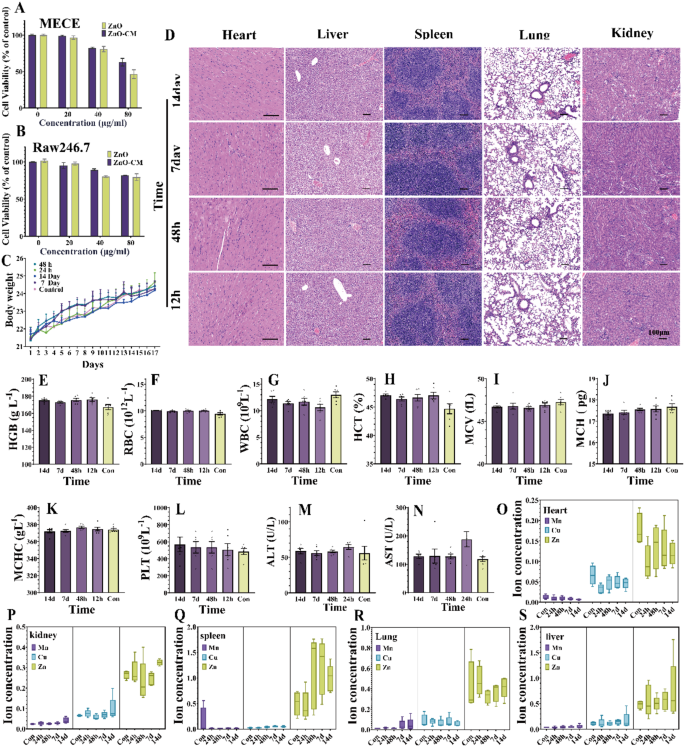
To make sure the sensible applicability of ZnO-CM, it’s important to completely assess its organic toxicity. Initially, the cytotoxicity of ZnO-CM was evaluated in each RAW 264.7 and MECE cells. The outcomes from the CCK-8 assay indicated that there was minimal cell loss of life in MECE cells at a ZnO focus of 20 µg/mL. At a focus of 40 µg/mL, cell viability was 80.79%, whereas ZnO-CM exhibited a viability of 82.33%. Nonetheless, when the focus elevated to 80 µg/mL, cell viability dropped to 46.43%, and the viability for ZnO-CM fell to 62.85%. These findings recommend that ZnO-CM reveals decrease cytotoxicity in comparison with ZnO at concentrations of as much as 80 µg/mL (Fig. 6A). Related outcomes are noticed in RAW 264.7 cells handled with ZnO-CM (Fig. 6B). Subsequently, the in vivo biocompatibility of ZnO-CM was completely evaluated in 8-week-old BALB/c mice. After 14 days of steady ZnO-CM administration, the physique weight of the mice continues to extend, with no vital variations noticed between the teams (Fig. 6C). Pathological examination reveals no necrosis, congestion, or hemorrhage within the coronary heart, liver, spleen, lung, or kidney in any of the teams (Fig. 6D). Entire blood evaluation outcomes (RBC, HGB, HCT, MCV, MCH, WBC and PLT) present no variations between the ZnO-CM handled group and the management group (Fig. 6E-L). Moreover, serum biochemical evaluation signifies that the liver perform parameters (AST and ALT) within the ZnO-CM remedy group are just like these within the management group (Fig. 6M, N), demonstrating its good biocompatibility with the liver.
Given the issues relating to elemental accumulation in visceral tissues, a standard concern related to metal-based nanocatalysts, we carried out ICP-MS testing on the hearts, livers, spleens, lungs, and kidneys of mice at totally different time factors to research the buildup of steel components. The outcomes reveal no vital variations within the ranges of Cu and Mn throughout the assorted metabolic organs as dosing period elevated (Fig. 6O-S). Nonetheless, Zn exhibited slight accumulation within the spleen after administration for a period of 48 h to 14 days in comparison with the management group. Apparently, as a substitute of a steady improve in collected Zn over time, a reducing development was noticed. No vital adjustments have been famous in different organs, suggesting that ZnO-CM is cleared comparatively rapidly in vivo with low potential for long-term toxicity.
preliminary security evaluation. (A) ZnO and ZnO-CM cytotoxicity of intestinal epithelial cells. (B) Cytotoxicity of ZnO and ZnO-CM on RAW 264.7 cells. (C) mice weight determine. (D) 24 h, 48 h after the remedy, 7 days and 14 days on coronary heart, liver, spleen, lung and kidney tissue histological analysis. (E–L) routine blood ranges of mice. (M.N) in mice serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ranges. (O-S) nanometer materials of Cu, Mn, Zn distribution in organs
Antibacterial and anti-inflammation analysis in vivo
We then investigated the antibacterial and anti-enteritis results of ZnO-CM in mice contaminated with Salmonella (Fig. 7A). The Mannequin group reveals a forty five% mortality price inside two days post-infection, whereas the ZnO group exhibits a lowered mortality price of 10%. Importantly, mice handled with both dose of ZnO-CM experiences no extra deaths over 4 days (Fig. 7B). Salmonella an infection ends in a 30% discount in physique weight, which is partially alleviated by ZnO (20% discount) and considerably improved by ZnO-CM (5% discount) (Fig. 7C). Moreover, Salmonella an infection results in vital colon shortening, indicative of colitis, which is notably alleviated by ZnO-CM remedy (Fig. 7D and E). To guage Salmonella distribution and the bactericidal efficacy of ZnO-CM, we contaminated mice with ST-RFP. In vivo imaging reveals Salmonella accumulation within the cecum and colon (Fig. 7F). ZnO remedy reduces Salmonella numbers by 98% within the small gut and 96% within the colon, whereas ZnO-CM achieves over a 99% discount, with the ZnO-CM-H group demonstrating practically full eradication (Fig. 7G and H). Immunofluorescence confirmed ZnO-CM’s in vivo effectiveness in killing Salmonella, paralleling in vitro outcomes (Fig. 7I). Hematoxylin and eosin (HE) staining demonstrates that each ZnO and ZnO-CM scale back Salmonella-induced colon injury, with ZnO-CM exhibiting a stronger protecting impact (Fig. 7J). The same protecting impact is noticed within the small gut (Fig. 7J). Salmonella an infection brought about irritation and cell loss of life, compromising the intestinal barrier perform, as evidenced by decreased ranges of tight junction proteins occludin and claudin-1, which have been restored by ZnO-CM remedy (Fig. S6). An infection activated NF-κB phosphorylation, growing expression of pro-inflammatory cytokines IL-1β and IL-6, however ZnO-CM considerably lowered these cytokines (Fig. 7Ok and L). Salmonella an infection additionally upregulated iNOS expression [40], whereas ZnO-CM lowered it, indicating its position in inhibiting M1 macrophage polarization and thereby relieving Salmonella-induced colitis (Fig. 6F-I) [41]. Due to this fact, ZnO-CM successfully reduces Salmonella-induced colon injury, weight reduction, and mortality in mice (Fig. 7B-E).
The antibacterial and anti inflammatory impact of ZnO-CM in vivo in a Salmonella-infected enteritis mannequin. Groups are divided into Management, Mannequin, ZnO (50 mg/kg), ZnO-CM-L (25 mg/kg), and ZnO-CM-H (50 mg/kg). (A) Schematic of the mouse mannequin institution and subsequent testing of antibacterial results within the colon and small gut. (B) Mortality curves (n = 20). (C) curves of physique weight in mice. (D) Comparability of colon size after remedy of Salmonella an infection. (E) Quantification of colon size. (F) ex vivo visceral imaging displaying bacterial focus and distribution. (G.H) teams of small gut and colon micro organism smear pictures and quantification after remedy. (I) immunofluorescence observations tissue part distribution of micro organism. (J) Hematoxylin-eosin (HE) staining of tissue sections of the small gut and colon. (Ok.L) expression ranges of inflammation-related proteins. n = 10, * p < 0.05, ** p < 0.01, *** p < 0.001