Superionic conducting mechanism
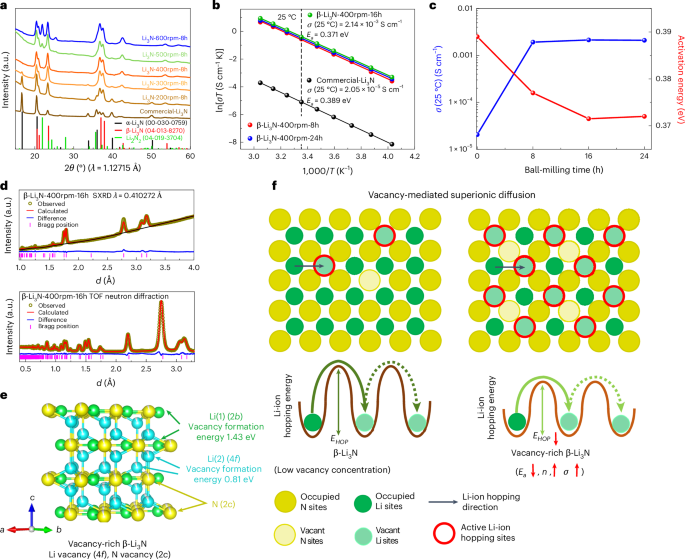
The ball-milling methodology is chosen to create excessive strain for acquiring pure β-Li3N from the business blended phased Li3N and a ball-milling pace of 400 rpm is chosen to arrange pure β-Li3N (Fig. 1a and Supplementary Word 1). Pure β-Li3N was obtained by ball milling at 400 rpm for 8 h, denoted by β-Li3N-400rpm-8h. The Arrhenius plots of the business Li3N and the β-Li3N-400rpm-8h are proven in Fig. 1b. The β-Li3N-400rpm-8h SSE demonstrated a excessive room-temperature (25 °C) ionic conductivity of 1.92 × 10−3 S cm−1, which is round two orders of magnitude larger than the business Li3N. The activation power (0.377 eV) for the β-Li3N-400rpm-8h SSE is decrease than that of business Li3N (that’s, 0.389 eV). When the ball-milling time additional will increase from 8 h to 16 h and 24 h with a relentless pace of 400 rpm, the ionic conductivity additional will increase. The corresponding β-Li3N SSEs ready by completely different ball-milling instances are denoted by β-Li3N-400rpm-16h and β-Li3N-400rpm-24h (Fig. 1b). A ball-milling time of 16 h results in an optimized ionic conductivity of two.14 × 10−3 S cm−1 at 25 °C and the bottom activation power of 0.371 eV (Fig. 1c). The achieved ionic conductivity is among the many highest values reported for not solely pure Li3N but additionally all nitride SSEs to date (Fig. 2a), displaying nice promise to realize excessive power and energy densities for all-solid-state lithium steel batteries21,34. Along with the section transformation, the development of ionic conductivity of the ball-milled β-Li3N in contrast with the business Li3N was associated to the vacancy-mediated lithium-ion diffusion mechanisms27.
a, The XRD patterns of the Li3N samples processed by completely different ball-milling speeds (the ball-milling time was 8 h for all samples). The commercially obtainable Li3N is often a combination of α- and β-phases. α-Li3N (house group P6/mmm) transforms into β-Li3N (house group P63/mmc) at an elevated strain. The ball-milling methodology (pace 400 rpm) is chosen to create excessive strain for acquiring pure β-Li3N from the business mixed-phased Li3N. b, Arrhenius plots of β-Li3N as a operate of the ball-milling time (the ball-milling pace is fixed of 400 rpm) and business Li3N. The lithium-ion conductivity of Li3N is evaluated by the alternating present (a.c.) impedance methodology utilizing pressed pellets. The plots evaluate the Arrhenius behaviour of business Li3N with β-Li3N samples milled at 400 rpm for various durations (8 h, 16 h and 24 h). c, Lithium-ion conductivity at 25 °C and activation power of β-Li3N plotted as capabilities of ball-milling length, carried out at a constant pace of 400 rpm. For comparability, business Li3N (with out ball milling) can also be introduced. This determine summarizes the room-temperature ionic conductivities and activation energies as a operate of ball-milling instances. d, SXRD patterns and TOF neutron diffraction information (financial institution 3) with corresponding Rietveld refinement outcomes for β-Li3N-400rpm-16h. The corresponding crystal buildings with a deal with lithium and nitrogen vacancies are refined utilizing the Rietveld methodology. e, Crystal construction of vacancy-rich β-Li3N and the calculated formation power of single impartial lithium emptiness at 2b and 4f websites (2b website, 1.43 eV; 4f website, 0.81 eV), respectively. f, Schematic illustration of vacancy-mediated superionic diffusion mechanism of vacancy-rich β-Li3N. EHOP, lithium-ion hopping power; Ea, activation power for lithium-ion conduction; n, focus of cell lithium ions; σ, ionic conductivity.
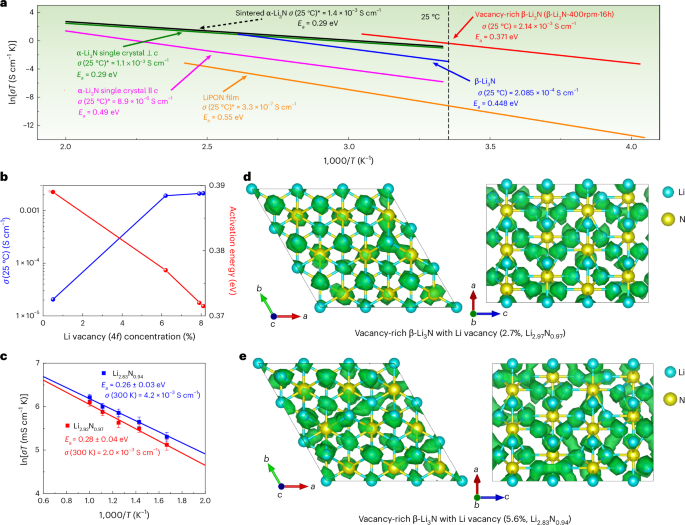
a, Arrhenius plots of vacancy-rich β-Li3N (that’s, β-Li3N-400rpm-16h) and different nitrides (α-Li3N single crystals39, α-Li3N sinter26, β-Li3N27 and LiPON movie42) for comparability. The room-temperature (25 °C) ionic conductivity is calculated based mostly on Arrhenius plots. For α-Li3N single crystals, the anisotropic lithium-ion conductivities parallel and perpendicular to the hexagonal c axis are introduced, denoted as α-Li3N single crystal || c, and α-Li3N single crystal ⊥ c, respectively. b, Lithium-ion conductivity at 25 °C and activation power of vacancy-rich β-Li3N as a operate of lithium emptiness focus (4f websites). c, Arrhenius plots of lithium-ion diffusivity in vacancy-rich β-Li3N with completely different lithium emptiness concentrations in AIMD simulations. The emptiness focus is 2.7% for Li2.92N0.97 and 5.6% for Li2.83N0.94. Statistical deviations in lithium-ion diffusivity have been evaluated because of the stochastic nature of ion hopping, with error bars representing commonplace deviations calculated from the full diffusional displacements and efficient ion hops noticed in AIMD simulations. d,e, Lattice buildings and superimposed lithium-ion chance density (marked by inexperienced iso-surfaces) in vacancy-rich β-Li3N with completely different lithium emptiness concentrations, 2.7% in Li2.92N0.97 (d) and 5.6% in Li2.83N0.94 (e), based mostly on AIMD simulations at 600 Okay.
The crystal buildings of business Li3N and ball-milled β-Li3N studied by SXRD and TOF neutron diffraction with a deal with lithium and nitrogen vacancies have been refined utilizing the Rietveld methodology, as proven in Fig. 1d,e, Supplementary Figs. 1–4 and Supplementary Tables 1–8. The business Li3N was decided as a combination of 63.1(9) wt% α-phase and 36.9(7) wt% β-phase, and the refined crystal buildings of the α- and β-phases are proven in Supplementary Fig. 1. Within the business Li3N, the Li(1) websites within the α-phase (1b) and the β-phase (2b) are absolutely occupied. Conversely, the Li(2) websites (2c in α-phase; 4f in β-phase) and N(3) websites (1a in α-phase; 2c in β-phase) are partially occupied. Subsequent calculations reveal that the lithium and nitrogen emptiness concentrations for each phases within the business Li3N are minimal: 0.7(4)% on the Li(2) 2c website (amounting to 0.5(4)% throughout all lithium websites) and 0.5(2)% on the N(3) 1a website within the α-phase, and 0.5(4)% on the Li(2) 4f website (equal to 0.3(4)% throughout all lithium websites) and 0.3(2)% on the N(3) 2c website within the β-phase. Within the case of the β-Li3N-400rpm-8h pattern, the purely constituted β-Li3N demonstrated an augmented lithium emptiness focus on the 4f website, roughly 6.2(6)% (translating to 4.1(6)% throughout all lithium websites) as indicated in Supplementary Fig. 2 and Supplementary Tables 3 and 4. The lithium and nitrogen emptiness concentrations within the vacancy-rich β-Li3N usually escalated with extended ball-milling durations, but plateaued submit 16 h. Particularly, the β-Li3N-400rpm-16h pattern, hereinafter known as vacancy-rich β-Li3N, showcased peak emptiness concentrations, with lithium vacancies approximated at 8.1(2)% on the Li(2) 4f websites (round 5.4(2)% for all lithium websites) and nitrogen vacancies at roughly 5.4(1)% on the N 2c websites (Fig. 1e). The presence of lithium vacancies on the 4f websites, reasonably than the twob websites, could be attributed to the comparatively weaker bonding between N3− and Li+ on the 4f sties, in addition to the low lithium emptiness formation power on the 4f website in contrast with the twob website (Fig. 1e and Supplementary Word 2).
Obvious correlations could be concluded that the upper lithium and nitrogen emptiness concentrations can result in the next focus of cell lithium ions, decrease lithium-ion diffusion obstacles (that’s, decrease activation power) and thus larger ionic conductivity as proven in Figs. 1f and 2b, Supplementary Fig. 5 and Supplementary Word 3. The lithium diffusion mechanism in vacancy-rich β-Li3N with completely different concentrations of lithium and nitrogen vacancies was additional studied by AIMD simulation. When vacancies have been launched, quick ionic conduction was noticed in a three-dimensional channel as proven in Fig. 2nd,e. The sooner ionic conduction was achieved for β-Li3N with the elevated emptiness focus in AIMD simulations (Fig. 2c). When complete emptiness concentrations elevated from 2.7% to five.6% in vacancy-rich β-Li3N, the activation power decreased from 0.28 ± 0.04 eV to 0.25 ± 0.03 eV with extrapolated lithium-ion conductivity elevated from 2.0 × 10−3 S cm−1 to 4.2 × 10−3 S cm−1 at 300 Okay. The results of accelerated lithium-ion diffusion within the crystal lattice by vacancies is according to our experimental outcomes that the ionic conductivity elevated from 2.05 × 10−5 S cm−1 to 2.14 × 10−3 S cm−1 when emptiness focus elevated from 0.5% to eight.1% at Li(2) 4f websites (from 0.3(4)% to five.4(2)% for complete lithium websites). The noticed enhancement in lithium-ion diffusion throughout the crystal lattice, attributed to the presence of vacancies, agrees with the experimental outcomes. Particularly, the ionic conductivity will increase from 2.05 × 10−5 S cm−1 to 2.14 × 10−3 S cm−1 because the emptiness focus on the Li(2) 4f websites elevated from 0.5% to eight.1% (translating to a rise from 0.3(4)% to five.4(2)% throughout all lithium websites). In response to the DFT calculations, the formation power of vacancy-rich β-Li3N throughout various concentrations will increase from 1.25 eV for Li2.92N0.97 (emptiness focus 2.7%) to 2.67 eV for Li2.83N0.94 (emptiness focus 5.6%) and 5.44 eV for Li2.67N0.89 (emptiness focus 11.1%) (Supplementary Fig. 6). This excessive formation power for the vacancy-rich β-Li3N might clarify the restrict for the achievable emptiness focus noticed in our experimental pattern, which exhibited a emptiness focus of roughly 5.4%. A deeper evaluation with scanning electron microscopy (SEM) probing the interrelationship between lithium-ion diffusion, particle dimension and emptiness focus pinpoints an elevated emptiness focus as an important determinant of enhanced lithium-ion diffusion throughout the β-Li3N SSEs (Supplementary Fig. 7 and Supplementary Word 4).
Chemical stability in direction of lithium steel and air
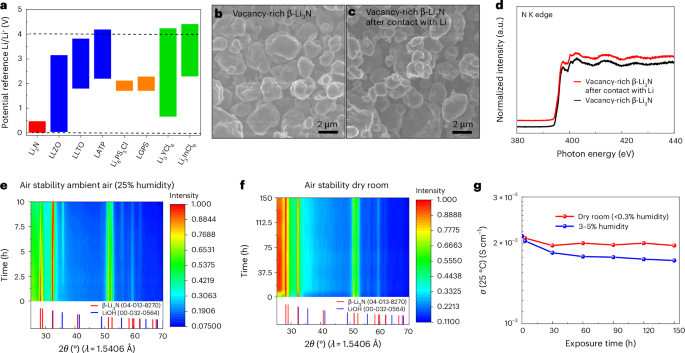
In response to the calculations (Fig. 3a and Supplementary Fig. 8), virtually all well-known SSEs are unstable with lithium steel owing to the discount of central cations. In contrast, solely vacancy-rich β-Li3N is secure towards lithium steel anode and reveals a secure electrochemical window of 0–0.48 V. In the meantime, as proven in Supplementary Fig. 9, the time-resolved electrochemical impedance spectroscopy (EIS) spectra of the Li/vacancy-rich β-Li3N/Li symmetric cell remained virtually unchanged for 30 h, confirming the thermodynamic stability of vacancy-rich β-Li3N in direction of lithium steel. SEM photographs, depicted in Fig. 3b,c, present the morphology of pristine and lithium-interacted vacancy-rich β-Li3N, revealing minimal morphological modifications and suggesting an absence of floor reactions with lithium steel. Ex situ X-ray absorption near-edge construction (XANES) evaluation additional corroborates the soundness of vacancy-rich β-Li3N SSE towards lithium, as illustrated in Fig. 3d. The N Okay-edge XANES spectrum characterizes electron transitions from nitrogen’s 1s orbital to the vacant digital states within the conduction band, with a notable peak at roughly 398 eV indicative of 1s to π* transitions and broader peaks round 400 eV and 403 eV similar to 1s to σ* transitions35,36. The consistency of nitrogen Okay-edge XANES spectra earlier than and after lithium contact underscores the SSE’s substantial chemical stability in direction of lithium steel. Enhanced spatial decision XANES spectra by means of STXM, proven in Supplementary Fig. 10a,b, additional validate these findings, displaying the preserved particle morphology of vacancy-rich β-Li3N upon lithium interplay. These STXM photographs, captured on the pre-edge of the N Okay-edge absorption (395 eV), and the nitrogen Okay-edge XANES spectra derived from the floor of β-Li3N particles post-lithium contact (information have been collected at photon energies spanning from 395 eV to 418 eV (refs. 37,38); Supplementary Fig. 10c), exhibit no notable chemical modifications, affirming the fabric’s resistance to reactions with lithium. The consistency throughout varied floor areas of a β-Li3N particle (Supplementary Fig. 10b) reinforces the absence of floor reactions, with solely minor spectral fluctuations attributed to the STXM approach’s photon power decision limits.
a, Calculated thermodynamics intrinsic electrochemical home windows of vacancy-rich β-Li3N and different frequent SSEs, together with oxides (that’s, La3Li7O12Zr2 (LLZO), 0.03–3.16 V; lithium lanthanum titanate (LLTO), 1.80–3.73 V; Li1.3Al0.3Ti1.7(PO4)3 (LATP), 2.19–4.20 V), sulfides (that’s, Li10GeP2S12 (LGPS), 1.71–2.29 V; Li6PS5Cl, 1.71–2.13 V) and halides (that’s, Li3YCl6, 0.65–4.25 V; Li3InCl6, 2.28–4.42 V). b,c, SEM photographs of the vacancy-rich β-Li3N (b) and the vacancy-rich β-Li3N pattern after contact with lithium (c). d, Normalized nitrogen Okay-edge XANES spectra of pristine vacancy-rich β-Li3N and the vacancy-rich β-Li3N pattern after contact with lithium. N Okay-edge XANES spectra have been collected within the TEY mode. e, Operando XRD sample evolution of vacancy-rich β-Li3N through the publicity course of to air with 25% relative humidity for 10 h, acquired at 30 min intervals over a ten h interval. f, In situ XRD sample evolution of vacancy-rich β-Li3N upon completely different publicity instances in a dry room with a low dew level of −50 °C to −60 °C (<0.3% relative humidity) for 150 h. Notably, the broad hump at roughly 26 levels 2θ corresponds to the Kapton tape used within the pattern preparation, which doesn’t exhibit distinct sharp XRD peaks. g, The lithium-ion conductivity evolution at 25 °C of vacancy-rich β-Li3N after completely different publicity instances in a dry room with a low dew level of −50 °C to −60 °C (<0.3% relative humidity) and ambient air with 3–5% humidity stage for 150 h.
Past its thermodynamic stability towards lithium steel, the air stability of vacancy-rich β-Li3N stands as one other important trait for sensible dealing with. To evaluate the air stability of the vacancy-rich β-Li3N pattern upon atmospheric publicity, we used operando and in situ X-ray diffraction (XRD) mixed with ex situ smooth X-ray XANES strategies, focusing on each the N Okay-edge and O Okay-edge. These strategies have been chosen provided that XRD gives sensitivity to crystal buildings, whereas smooth X-ray XANES collected within the complete electron yield (TEY) mode gives insights into floor chemistry. Determine 3e and Supplementary Fig. 11 depict operando XRD outcomes for the vacancy-rich β-Li3N pattern when uncovered to ambient air with a relative humidity of 25%. Notably, the emergence of LiOH impurity was noticed roughly 1–1.5 h submit air publicity, attributable to its interplay with atmospheric moisture, as inferred from the operando XRD and ex situ XANES research (Supplementary Figs. 11a and 12). After the ten h publicity length, the dominant β-Li3N section remained largely intact, accompanied by a constant proportion of the LiOH impurity. It’s postulated that the formation of LiOH acted as a protecting barrier, limiting the additional publicity of β-Li3N to moisture, as corroborated by the operando XRD and ex situ XANES analyses (Supplementary Figs. 11a and 12). Analogous observations have been documented for α-Li3N single crystals39. The chemical resilience of the vacancy-rich β-Li3N pattern underneath dry rooms (dew factors −50 °C to −60 °C, equal to <0.3% relative humidity) is ascertained by way of in situ XRD and ex situ XANES. Determine 3f and Supplementary Fig. 11b present the in situ XRD information for samples saved for as much as 150 h in a dry room atmosphere. Noteworthy is the broad hump round 26 levels 2θ, attributed to Kapton tape used throughout pattern preparation, absent of distinct, sharp XRD peaks. Notably, these samples constantly exhibited the β-Li3N section with uniform peak intensities and confirmed no extra peaks suggestive of crystalline LiOH or different secondary crystalline phases. Moreover, the ex situ XANES information, depicted in Supplementary Fig. 12c,d (Supporting Info), confirm the presence of the LiOH layer on the β-Li3N floor. This LiOH layer probably acts as a protecting barrier, mitigating additional publicity of the β-Li3N to moisture. Determine 3g compares the lithium-ion conductivity of vacancy-rich β-Li3N at 25 °C after completely different atmospheric/humidity exposures. Excessive ionic conductivities above 10−3 S cm−1 have been maintained after completely different publicity circumstances, even for the pattern uncovered to three–5% humidity for 150 h. The findings point out that β-Li3N displays stability underneath circumstances of low humidity, each in a dry room atmosphere and through extended storage, rendering it appropriate for integration into large-scale industrial manufacturing processes. Nonetheless, publicity to high-humidity environments and direct contact with water current potential hazards for this vacancy-rich β-Li3N. To mitigate these security considerations, it’s advisable to implement coating methods designed to reduce dangers40. The standards for choosing acceptable coating supplies embrace compatibility with vacancy-rich β-Li3N, resistance to moisture intrusion, excessive ionic conductivity and minimal digital conductivity.
All-solid-state lithium steel batteries
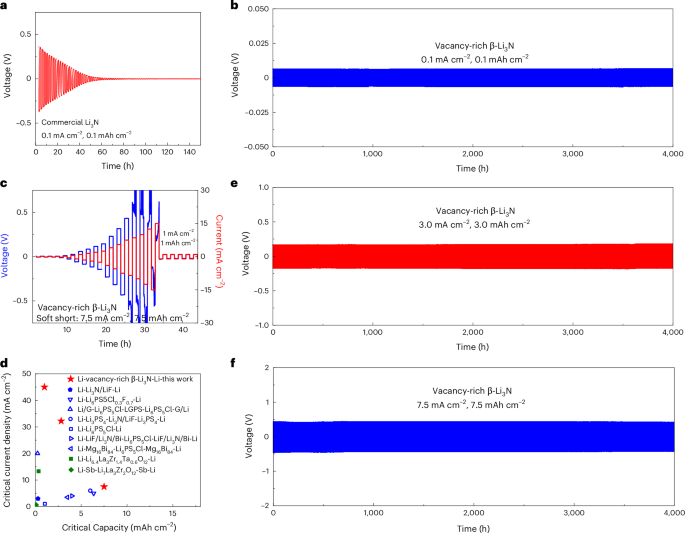
Decided by the direct present polarization methodology (Supplementary Fig. 13), this vacancy-rich Li3N reveals a low digital conductivity of ~4.5 × 10−10 S cm−1, which was promising to make sure secure lithium stripping and plating behaviour even at excessive present densities and capacities (Supplementary Word 5). All-solid-state lithium symmetric cells have been fabricated to research the lithium stripping and plating behaviours, as depicted in Supplementary Fig. 14. To evaluate the efficiency of commercially obtainable Li3N, an all-solid-state Li/commercial-Li3N/Li symmetric cell was evaluated at present densities and capability of 0.1 mA cm−2 and 0.1 mAh cm−2. Determine 4a reveals an preliminary overpotential of roughly 0.5 V, attributable to the low ionic conductivity (particularly, 2.05 × 10−5 S cm−1). In subsequent stripping/plating cycles, this overpotential quickly decreased to just about 0 V inside 80 h, suggesting critical lithium dendrite progress throughout the business Li3N SSE layer and a heightened propensity for short-circuiting. In stark distinction, the all-solid-state Li/vacancy-rich β-Li3N/Li symmetric cell showcased excellent electrochemical efficiency underneath similar biking circumstances. Determine 4b and Supplementary Figs. 15, 16 and 17a elucidate that each the preliminary overpotential and the EIS spectra of this symmetric cell remained constant even after 4,000 h of biking. Determine 4c demonstrates the cell’s secure potential profiles, enduring a notable present density as much as 7.5 mA cm−2 and a commendable capability of seven.5 mAh cm−2. Whereas the overpotential profiles manifested fluctuations between 7.5 and 15 mA cm−2 – which suggests potential lithium dendrite formation underneath these rigorous circumstances – the constant subsequent stripping/plating efficiency at 1 mA cm−2 and 1 mAh cm−2 indicated the non-existence of a tough quick circuit. The Li/vacancy-rich β-Li3N/Li symmetric cell showcased a transient adaptability to heightened present densities and capacities, withstanding as much as 15 mA cm−2 and 15 mAh cm−2, respectively41. After this rigorous evaluation, steady biking at 1 mA cm−2 and 1 mAh cm−2 continued for 3,000 h, as introduced in Supplementary Fig. 18. Analyses by way of EIS, XANES and SEM analyses collectively additional verify the distinctive stability underneath excessive present densities, as evident in Supplementary Fig. 9 and Supplementary Word 6. For preset essential capacities of 1 mAh cm−2 and three mAh cm−2, peak essential present densities of 45 mA cm−2 and 32.5 mA cm−2 have been documented, as illustrated in Supplementary Figs. 18 and 19. In contrast with present all-solid-state lithium symmetric cells using sulfide, nitride and oxide SSEs, and interlayer design, the Li/vacancy-rich β-Li3N/Li symmetric cell exhibited substantial enhancements in essential present densities and biking capacities, as concisely illustrated in Fig. 4d. For the pursuit of excessive power and energy densities, prolonged lithium stripping/plating biking was evaluated at varied circumstances. Determine 4e,f and Supplementary Fig. 15 exhibit that the Li/vacancy-rich β-Li3N/Li symmetric cells maintained constant biking efficiency over 4,000 h at each 3 mA cm−2 (capability 3 mAh cm−2) and seven.5 mA cm−2 (capability 7.5 mAh cm−2). As well as, Supplementary Figs. 17 and 19 showcase thinner layers of vacancy-rich β-Li3N (15 mg, roughly 0.25 mm), highlighting the significance of minimizing overpotential within the Li/vacancy-rich β-Li3N/Li symmetric cells for sensible utility in full cells. The cells exhibited low overpotentials and sustained biking efficiency throughout 750 h at present densities of 0.1 mA cm−2 (capability 0.1 mAh cm−2) and seven.5 mA cm−2 (capability 7.5 mAh cm−2), and secure biking by means of 500 cycles at 45 mA cm−2 (capability 1 mAh cm−2) and 32.5 mA cm−2 (capability 3 mAh cm−2), together with secure biking over 500 cycles at each 45 mA cm−2 (and 1 mAh cm−2) and 32.5 mA cm−2 (and three mAh cm−2). Moreover, Supplementary Fig. 20 showcases the exceptional biking stability of the Li/vacancy-rich β-Li3N/Cu uneven all-solid-state cells throughout 1,000 cycles at varied circumstances: 0.1 mA cm−2 and 0.1 mAh cm−2, 3 mA cm−2 and three mAh cm−2, and eventually, at 7.5 mA cm−2 and seven.5 mAh cm−2.
a,b, Voltage profiles of Li/commercial-Li3N/Li (a) and Li/vacancy-rich β-Li3N/Li (b) symmetric all-solid-state cells with a present density of 0.1 mA cm−2 and a set capability of 0.1 mAh cm−2. c, Voltage profiles of the Li/vacancy-rich β-Li3N/Li symmetric all-solid-state cell with incremental present densities and capacities (lithium plating/stripping for fastened 1 h) adopted by a set present density of 1 mA cm−2 and a set capability of 1 mAh cm−2. Given the paramount significance of essential present densities and capability in attaining elevated power and energy densities, the symmetric cell underwent evaluation with progressively growing present densities, all of the whereas preserving a relentless stripping/plating length of 1 h. d, Comparability of the essential present densities and capability for lithium symmetric cells of this work and different stories utilizing sulfide-, oxide- and nitride-based SSEs: Li-Li6PS5Cl0.3F0.7-Li43, Li/G-Li6PS5Cl-LGPS-Li6PS5Cl-G/Li2, Li-Li3PS4-Li3N/LiF-Li3PS4-Li33, Li-Li3N/LiF-Li33, Li-Li6PS5Cl-Li12, Li-LiF/Li3N/Bi-Li6PS5Cl-LiF/Li3N/Bi-Li18, Li-Mg16Bi84-Li6PS5Cl-Mg16Bi84-Li19, Li-Li6.4La3Zr1.4Ta0.6O12-Li44, Li-Sb-Li7La3Zr2O12-Sb-Li45. e,f, Voltage profiles of the Li-vacancy-rich β-Li3N-Li symmetric all-solid-state cell with a present density of three mA cm−2 and a set capability of three mAh cm−2 (e) and a present density of seven.5 mA cm−2 with a set capability of seven.5 mAh cm−2 (f). Information introduced on this determine have been obtained utilizing all-solid-state pellet-type cells.
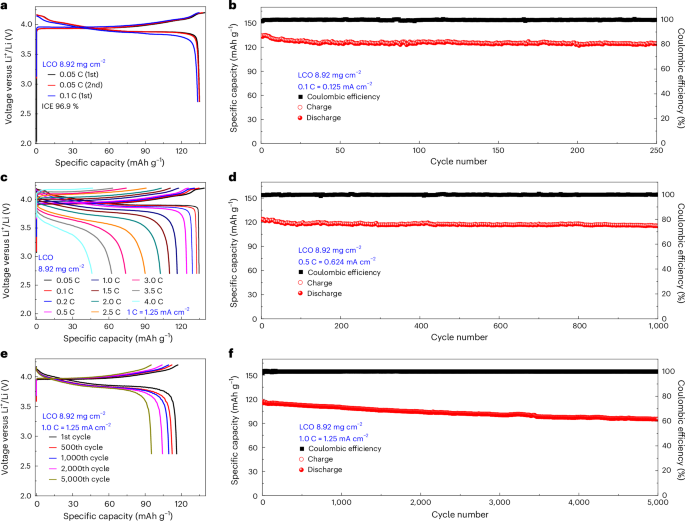
All-solid-state lithium steel batteries have been fabricated with LiCoO2 (LCO) and Ni-rich LiNi0.83Co0.11Mn0.06O2 (NCM83) serving as cathodes. Halide SSEs, Li3InCl6 and Li3YCl6, have been paired with this vacancy-rich β-Li3N to kind the SSE layer, whereas lithium steel constituted the anode, leading to LCO or NCM83/Li3InCl6/Li3YCl6/vacancy-rich β-Li3N/Li full cells (Supplementary Figs. 14 and 21, and Supplementary Word 7). The LCO/Li3InCl6/Li3YCl6/vacancy-rich β-Li3N/Li full cells with an LCO loading of 8.92 mg cm−2 exhibit secure biking efficiency (Fig. 5 and Supplementary Fig. 22). The total cell exhibited a formidable preliminary discharge capability of 139.2 mAh g−1 coupled with a excessive preliminary Coulombic effectivity (ICE) of 96.9% at a charge of 0.05 C. At a charge of 0.1 C, the cell achieved a reversible capability of 133.3 mAh g−1 and maintained an elevated Coulombic effectivity of 99%. Notably, the discernible phase-transition-induced voltage plateaus alluded to minimal polarization, making certain environment friendly Li⁺ ion transport throughout the LCO and SSE interface. As depicted in Fig. 5c, at biking charges of 0.5 C, 1.0 C, 2.0 C, 3.0 C and 4.0 C, the reversible capacities have been sustained at 124 mAh g−1, 116 mAh g−1, 102 mAh g−1, 73 mAh g−1 and 46 mAh g−1, respectively. The compatibility of the lithium steel with the vacancy-rich β-Li3N layer is additional underscored by the extended biking lifetime of the complete cell, as illustrated in Fig. 5b,d–f. When subjected to a biking charge of 0.1 C, the cell demonstrated constant capability retention, delivering 124 mAh g−1 throughout 250 cycles. At larger present densities of 0.5 C and 1.0 C, wonderful long-term biking stability with excessive reversible capability (115.5 mAh g−1 over 1,000 cycles at 0.5 C and 95.21 mAh g−1 over 5,000 cycles at 1.0 C) and excessive capability retention (93.6% over 1,000 cycles at 0.5 C and 82.05% over 5,000 cycles at 1.0 C) have been demonstrated. Supplementary Fig. 22 (Supporting Info) illustrates the typical biking efficiency at charges of 0.1 C, 0.5 C and 1.0 C throughout ten cells for every situation, confirming the reproducibility and robustness of the all-solid-state lithium steel cells’ efficiency.
Lengthy-term electrochemical efficiency of the LCO/Li3InCl6/Li3YCl6/vacancy-rich β-Li3N/Li all-solid-state lithium steel batteries with an LCO loading of 8.92 mg cm−2 at 25 °C with an working voltage window between 4.2 V and a pair of.7 V. a,c,e, The cost/discharge curves at 0.05 C and 0.1 C (a) and incremental biking charges as much as 4.0 C (c) and 1.0 C (e) (for the first, five hundredth, 1,000th, 2,000th and 5,000th cycles). b,d,f, Cost–discharge capability and the Coulombic effectivity as a operate of cycle quantity for all-solid-state lithium steel batteries cycled at 0.1 C (b), 0.5 C (d) and 1.0 C (f). Information introduced on this determine have been obtained utilizing all-solid-state pellet-type cells.
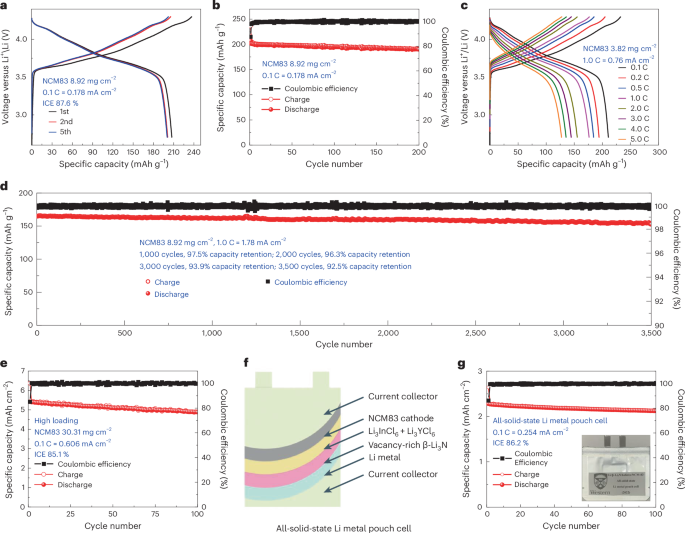
Determine 6a presents the voltage profiles of the NCM83/Li3InCl6/Li3YCl6/vacancy-rich β-Li3N/Li full cells at a charge of 0.1 C (the place 1 C = 200 mA g−1). The ICE registers at 87.6%, and the Coulombic effectivity of subsequent cycles approaches roughly 100%, as depicted in Fig. 6b, Supplementary Fig. 23 and Supplementary Word 8. The reversible capability of full cells was ~207 mAh g−1 after which maintained at ~195 mAh g−1 and ~190 mAh g−1 over 100 and 200 cycles, respectively. One other full cell with an NCM83 loading of three.82 mg cm−2 achieved quick charging and discharging efficiency as much as 5.0 C and might attain 83.77%, 73.90%, 68.63%, 64.31% and 60.47% of the reversible capability at 1.0 C, 2.0 C, 3.0 C, 4.0 C and 5.0 C, respectively (Fig. 6c and Supplementary Fig. 24). We additionally demonstrated ultra-long biking lifetime of all-solid-state lithium steel batteries as proven in Fig. 6d. At 1.0 C, the complete cell introduced a excessive reversible capability of ~142 mAh g−1 and an ultra-high capability retention of 92.5% over 3,500 cycles, suggesting excessive chemical stability and excessive compatibility of vacancy-rich β-Li3N layers to lithium steel throughout lengthy biking life. The total cells with a considerable NCM83 loading of 30.31 mg cm−2 exhibited a formidable preliminary areal capability of 5.42 mAh cm−2. They achieved a commendable ICE of 85.1% and sustained a sturdy areal capability of roughly 4.88 mAh cm−2 over 100 cycles (Fig. 6e). Supplementary Fig. 25 particulars the typical biking efficiency of full cells with an NCM83 loading of 8.92 mg cm−2 at 0.1 C and 1.0 C, in addition to these with a excessive areal loading of 30.31 mg cm−2, based mostly on testing 10 cells for every biking situation, demonstrating excessive reproducibility for the all-solid-state lithium steel cells. Given the pragmatic calls for of electrical autos (EVs), all-solid-state lithium steel pouch cells emerge as a potent method, aiming for an elevated power density (approaching 500 Wh kg−1) and making certain an prolonged driving vary (surpassing 300 miles) for EVs. All-solid-state lithium steel pouch cells are fabricated by means of the dry-film approach (Fig. 6f,g and Supplementary Fig. 26). Notably, this all-solid-state lithium steel pouch cell introduced a exceptional ICE of 86.2% and sustained a capability nearing 2.11 mAh cm−2 throughout 100 cycles. The common biking efficiency of those all-solid-state pouch cells, demonstrating their excessive reproducibility, is documented in Supplementary Fig. 25.
Lengthy-term electrochemical efficiency of the NCM83/Li3InCl6/Li3YCl6/vacancy-rich β-Li3N/Li all-solid-state lithium steel batteries at 25 °C with an working voltage window between 4.3 V and a pair of.7 V and the NCM83 loading of 8.92 mg cm−2 and three.82 mg cm−2. a,c, The cost/discharge curves at 0.1 C (a) and incremental biking charges as much as 5.0 C (c). b,d, Cost–discharge capability and the Coulombic effectivity as a operate of cycle quantity for all-solid-state lithium steel batteries cycled at 0.1 C (b) and 1.0 C (d). e, Cost–discharge capability and Coulombic effectivity as a operate of cycle variety of excessive loading all-solid-state lithium steel batteries efficiency (areal loading of NMC83, 30.31 mg cm−2; preliminary reversible areal capability, 5.42 mAh cm−2). Information introduced in a–e have been obtained utilizing all-solid-state pellet-type cells. f,g, Schematic (f) and cost–discharge capability and Coulombic effectivity (g) as a operate of cycle variety of an all-solid-state pouch cell with a excessive areal capability (preliminary reversible areal capability 2.28 mAh cm−2). The dry-film approach is adopted to assemble these all-solid-state lithium steel pouch cells. An NCM83 cathode movie is developed, encompassing a combination of NCM83 and Li3InCl6 SSE, and providing a reversible areal capability of round 2.28 mAh cm−2. Halide SSE movies, integrating Li3InCl6 and Li3YCl6 SSEs, alongside a vacancy-rich β-Li3N movie, are fabricated as detailed in Supplementary Fig. 27. These crafted parts have been systematically layered and pressurized along with a lithium steel foil, culminating in a complete all-solid-state full cell. This meticulously assembled full cell was then hermetically sealed inside an aluminium pouch in a vacuum atmosphere, showcased in g.
The secure electrochemical efficiency noticed in all-solid-state lithium steel batteries could be attributed to a number of key elements: the excellent structural resilience and lithium steel compatibility of the vacancy-rich β-Li3N SSE all through the electrochemical biking, the sturdy interfacial stability throughout the layered SSE structure and the efficient compatibility of Li3InCl6 with the used cathode supplies. The structural stability of this vacancy-rich β-Li3N SSE throughout electrochemical biking is confirmed by ex situ SXRD, XANES, SEM and EDX mapping outcomes, as depicted in Supplementary Figs. 27–38, Supplementary Tables 9–12 and Supplementary Word 9. To additional prolong the examine’s scope to a broader vary of functions, extra commercially obtainable SSEs resembling Li6PS5Cl are investigated. In all-solid-state lithium steel battery configurations comprising LCO or NCM83/Li6PS5Cl/LYC/β-Li3N/Li, exceptional electrochemical stability is noticed at each 0.1 C and 1 C biking charges as proven in Supplementary Fig. 39 and Supplementary Word 10. To handle the essential requirement for air stability in SSEs for sensible functions, the vacancy-rich β-Li3N SSEs, after publicity to ambient air for 10 h (hereafter known as β-Li3N-air-10h), nonetheless exhibit excessive lithium-ion diffusion traits, good electrochemical efficiency and sturdy interfacial stability, as depicted in Supplementary Figs. 40–43 and Supplementary Word 11. Contemplating price as a essential issue for sensible utility, the expense related to β-Li3N SSE has been evaluated towards that of different standard sulfide, halide and oxide SSEs, as depicted in Supplementary Fig. 44. This comparability reveals that the price of β-Li3N SSE is on par with that of extensively used business SSEs, resembling Li6PS5Cl, Li3InCl6, La3Li7O12Zr2 (LLZO) and Li6.4La3Zr1.4Ta0.6O12 (LLZTO). Moreover, it’s anticipated that the price of β-Li3N SSE may lower considerably with the adoption of large-scale manufacturing processes, underscoring its potential viability for the battery trade at massive. In abstract, the demonstrated excessive ionic conductivity at ambient temperature, distinctive biking efficiency, substantial interfacial stability and low price collectively spotlight the exceptional air stability and huge potential for sensible functions of the vacancy-rich β-Li3N SSE.