Synthesis of polymers and characterization of nanoparticles
To attain an excellent drug supply system able to encapsulating each chemotherapeutic medication and proteins, we synthesized a polyethyleneimine-oleic acid polymer advanced (PEI-OA) by conjugating OA to the carboxyl teams of PEI (molecular weight 1800 Da). The response method and 1H-NMR spectra are introduced in Fig. 2a and Fig. S1. The 1H-NMR outcomes revealed a attribute peak at δ 8.97 ppm, indicating the profitable synthesis of PEI-OA. OCT, an artificial octapeptide by-product, can goal the SSTR2 receptor on hepatocellular carcinoma cells. Given the potential concentrating on impact of OCT, we synthesized LNA-PEG-OCT by conjugating OCT to LNA-PEG-NHS through a Michael addition response. The response method, 1H-NMR, and infrared spectra outcomes are introduced in Fig. 2b and Fig. S2. The disappearance of the maleimide proton (4.45 ppm) in LNA-PEG-OCT in comparison with LNA-PEG-NHS confirms the profitable coupling of octreotide to LNA-PEG-NHS.
As a management group, positively charged nanoparticles (NPs) have been shaped utilizing the thin-film hydration technique and have been composed of PEI-OA, Soloplus®, and HS15. Negatively charged nanoparticles (CS/NPs) have been obtained by including CS to the NPs. Focused nanoparticles (OCT/CS/NPs) have been obtained by including LNA-PEG-OCT to CS/NPs through the preparation course of (Fig. 2c). Transmission electron microscopy outcomes confirmed that NPs and OCT/CS/NPs have been uniform and spherical. In distinction, OCT/CS/NPs had an additional layer of movie on their surfaces in comparison with NPs. The scale of OCT/CS/NPs was clearly greater than NPs (Fig. 2d), which can be attributed to the coating of the polymer compound CS. NPs had a constructive zeta potential of 27.3 mV and a particle dimension of 66.89 nm, whereas OCT/CS/NPs had a detrimental zeta potential of 28.5 mV and a particle dimension of 198.7 nm as a result of anionic CS polymer coating. To check the soundness of the nanoparticles at room temperature, we recorded the particle dimension, floor cost, and polymer dispersity index of NPs and OCT/CS/NPs over a interval of 48 h utilizing Dynamic Mild Scattering (DLS). As proven in Fig. 2e and f, the particle dimension of NPs modified from 66.89 to 69.9 nm, and the zeta potential modified from 27.3 to 24 mV. The particle dimension of OCT/CS/NPs modified from 198.3 to 205.7 nm, and the zeta potential modified from − 28.5 to -26.8 mV. This indicated that each NPs and OCT/CS/NPs have been steady sufficient to ensure focused supply in vivo. To research the affect of nanoparticles on the protein corona in blood, we carried out a serum stability evaluation. As illustrated in Fig. S3, the nanoparticles maintained constant cost and dimension all through their interplay with fetal bovine serum, indicating a minimal propensity for forming a protein corona. Moreover, analysis signifies that nanoparticles sized between 10 and 200 nm can evade renal and reticuloendothelial system (RES) clearance whereas selling optimum extravasation at tumor websites. Nanoparticles with barely detrimental or impartial floor costs are thought-about optimum for mitigating protein corona results [45, 46].
To judge the injection security of the OCT/CS/NPs, their hemolytic exercise was investigated. As proven in Fig. 2g, no important hemolytic exercise was detected, even at a focus of 10 g/L. In distinction, the hemolytic exercise of the NPs with out CS was considerably larger than that of the OCT/CS/NPs (Fig. S4).
To research the drug launch habits of nanoparticles in vitro, we first ready the usual curve of PTX utilizing a UV spectrophotometer (Fig. S5). The cumulative launch fee of PTX-Sol, PTX-NPs, and PTX-OCT/CS/NPs at totally different time factors was calculated (Fig. 2h). 4 days after launch, PTX within the PTX-OCT/CS/NPs group launched 16.94% lower than in PTX-NPs and 29.55% lower than in PTX-Sol. The cumulative launch fee of PTX from the PTX-Sol group reached as much as 85%, whereas it was 58.95% for PTX-OCT/CS/NPs. This means a sure sustained launch impact of PTX-OCT/CS/NPs. The discharge of PTX from nanoparticles primarily takes place via a diffusion mechanism, the place PTX molecules migrate from the inside of the particle, which has a excessive focus of PTX, to the encircling surroundings the place their focus is decrease.
To confirm the encapsulation means of NPs for RNP, we added RNP to the NP resolution underneath gradual vortexing at 37 °C and carried out the related characterizations. As proven in Fig. 2i, the particle dimension and zeta potential of PTX + RNP-OCT/CS/NPs didn’t change considerably after including RNP to PTX-OCT/CS/NPs. Fig. S6 exhibits that the loading of RNPs in PTX + RNP-OCT/CS/NPs (90%) was increased than that of PTX + RNP-NPs (87%), through the use of free sgRNA as a management.
Development and characterization of multifunctional nanoparticles. (a) Chemical construction of the polyethyleneimine-oleic acid (PEI-OA) polymer. (b) Chemical construction of the LNA-PEG-OCT polymer. (c) Transmission electron micrographs (TEM) of NPs and OCT/CS/NPs. Scale bar, 200 nm. (d) Zeta potential of PTX-NPs and PTX-OCT/CS/NPs. (e) Polydispersity index of PTX-NPs and PTX-OCT/CS/NPs at totally different time factors. (f) Measurement of PTX-NPs and PTX-OCT/CS/NPs at totally different time factors. (g) Hemolytic evaluation of PTX-NPs and PTX-OCT/CS/NPs. (h) In vitro drug launch of PTX from PTX-NPs and PTX-OCT/CS/NPs. (i) Characterisation of NPs after encapsulation of RNPs. All information are represented as means ± SD, n = 3
Endocytosis and endonucleolytic cleavage exercise of Cas9 RNP in vitro
To visualise the mannequin drug, we employed the near-infrared fluorescent dye DiD instead of PTX, and the fusion protein of enhanced inexperienced fluorescent protein and Cas9 (EGFP-Cas9) to switch the Cas9 protein. We in contrast the mobile uptake effectivity of PBS, DiD and EGFP-Cas9 resolution (DiD + EGFP-Cas9-Sol), DiD and EGFP-Cas9 loaded positively charged nanoparticles (DiD + EGFP-Cas9-NPs), DiD and EGFP-Cas9 loaded negatively charged nanoparticles (DiD + EGFP-Cas9-CS/NPs), and DiD and EGFP-Cas9 loaded focused nanoparticles (DiD + EGFP-Cas9-OCT/CS/NPs) utilizing confocal microscopy. The business transfection reagent Lipofectamine 2000 (DiD + EGFP-Lip2000) was used as a management to match the transfection effectivity of various formulations (Fig. 3a and S7). As seen within the determine, after 6 h of transfection, all nanoparticle teams had entered the inside of the cell. The cells handled with DiD + EGFP-Cas9-OCT/CS/NPs exhibited essentially the most EGFP-Cas9 round and contained in the nucleus in comparison with different teams, indicating that the synergistic dual-targeting position of OCT and CS aids within the supply of extra EGFP-Cas9 into the nucleus of the cell. This extremely environment friendly nucleus-targeted accumulation of DiD + EGFP-Cas9-OCT/CS/NPs will facilitate Cas9’s gene-editing perform within the nucleus. Furthermore, OCT/CS/NPs delivered essentially the most DiD into the cells in comparison with CS/NPs, NPs, and Lip2000.
We handled cells with DiD + EGFP-RNP-OCT/CS/NPs and analyzed their fluorescence by move cytometry to detect the encapsulation effectivity of OCT/CS/NPs for biotechnology medication with massive molecules and chemical medication with small molecules (Fig. 3b). The overlapping fluorescence of EGFP-RNP and DiD indicators within the OCT/CS/NPs confirmed that OCT/CS/NPs may encapsulate each chemotherapeutic medication and proteins concurrently, serving as a co-delivery platform for chemotherapy and gene remedy.
Lysosome degradation is a significant impediment throughout Cas9 protein supply. Whether or not the OCT/CS/NPs supply system can defend the Cas9 protein from enzymes within the lysosome and obtain lysosome escape is essential. Cells have been handled with totally different formulations. After 6 h of incubation, we stained lysosomes with a inexperienced fluorescent probe and noticed them utilizing a laser scanning confocal microscope (Fig. 3c and S8). Confocal microscope photographs demonstrated that each the DiD-OCT/CS/NPs and EGFP-OCT/CS/NPs teams underwent a definite endocytosis and endosomal escape course of. Initially, the pink indicators of DiD and the inexperienced indicators of endocytosis colocalized, whereas the inexperienced indicators of EGFP-Cas9 and the pink indicators equally colocalized. Over a interval of 6 h, these indicators steadily separated. Notably, this separation was extra pronounced within the DiD-OCT/CS/NPs and EGFP-Cas9-OCT/CS/NPs teams in comparison with the EGFP-Cas9-NPs and DiD-NPs teams. The outcomes proved that, as a result of Proton Sponge Impact of PEI-OA and the CS coating, PTX + RNP-OCT/CS/NPs can defend the Cas9 protein from lysosomal digestion.
Current research have proven that PD-L1 performs an amazing position in most cancers cells by binding to the receptor PD-1 on T cells, thus inhibiting the anti-tumor immunity of T cells [47]. After that, the most cancers cells will escape from immune system and proliferate quickly [48]. Right here, we used Snapgene software program and LentiCRISPR V2 plasmid to assemble a sgRNA (Fig. S9) that may goal PD-L1 (sgPD-L1). After PCR amplification and transcription, agarose gel electrophoresis confirmed focused cleavage means of sgPD-L1 on PD-L1 gene in vitro (Fig. 3d, S10,S11 and Desk. S1). To additional confirm the concentrating on and modifying means of RNP shaped by sgPD-L1 and Cas9 protein we designed, we extracted the genome from HepG2 cells and constructed a plasmid by T5-enzymatic concatenation with PUC18 vector (Fig. 3e, S12 and Desk. S2). We utilized the gene of STAT3 for the management assay, and ready two totally different sequence of sgPD-L1. Outcomes confirmed that RNP2 (shaped by Cas9 protein and sgPD-L1-1) had a greater cleavage effectivity than RNP1 (shaped by Cas9 protein and sgPD-L1-2) on PD-L1 gene contained plasmid. RNP2 was chosen to do the next experiments. To search out essentially the most appropriate ratio of Cas9/sgPD-L1, we carried out in vitro cleavage assay on plasmid with totally different ratios of RNPs (Fig. 3f), and the outcomes proved that one of the best molar ratio of RNP complexes was 1:3. Moreover, totally different ratios of RNP loaded nanoparticles have been utilized to HepG2 cells planted 96-well plates. After 24 hours’ incubation, cell proliferation was examined utilizing the MTT assay (Fig. S13). The outcomes additional confirmed that the RNP ratio of 1:3 had the strongest cytotoxicity on most cancers cells, so 1:3 was chosen to do the following experiments.
The gene for modifying PD-L1 is a 609 bp-long sequence and situated within the genome, and the modifying website is on the sequence of GTGAAATTGCAGGATGCAG. To validate the concentrating on and modifying means of the RNP, we in contrast totally different formulations (PBS, PTX-Sol, PTX + RNP-NPs, PTX + RNP-OCT/CS/NPs) on HepG2 cells and examined by T7 nucleic acid endonuclease I (T7EI). Determine 3g confirmed that the modifying effectivity of the PTX + RNP-OCT/CS/NPs group reached 55.8% indicating gene modifying impact.
To confirm the modifying means of Cas9 protein we designed and purified, we additionally designed appropriate sgRNA for GFP gene (sgGFP) and evaluated the gene modifying means of GFP RNP in inexperienced florescent protein stably expressed 293T cells (GFP-293T). Leads to Fig. 4a confirmed that, after 72 h incubation, the fluorescence of RNP-NPs and RNP-OCT/CS/NPs was decreased considerably in comparison with the opposite teams. GFP-293T handled with RNP-OCT/CS/NPs has the least fluorescence in contrast with RNP-Sol, RNP-NPs and RNP-Lipo2000. It was indicated that RNP-OCT/CS/NPs realized a profitable modifying of the GFP gene.
Validation of sgRNA composition and Cas9 protein exercise. (a) Endocytosis of EGFP and DiD into HepG2 cells by totally different nanoparticles for six h. Scale bar, 100 nm. (b) Stream cytometry of DiD-OCT/CS/NPs, FITC-BSA-OCT/CS/NPs, and DiD + FITC-BSA-OCT/CS/NPs. (c) Investigation on the lysosomal escape means of various nanoparticles. (d) Eight sgRNAs synthesized by in vitro transcription (sgPD-L1: 1 ~ 4, sgSTAT3: 5–8). (e) 1% agarose gel electrophoresis was used to detect the effectivity of in vitro cleavage of the puc18-PD-L1 plasmid by RNPs. (f) The in vitro cleavage effectivity of various ratios of Cas9 RNP (Cas9:sgPD-L1, mol: mol) was examined by 2% agarose gel electrophoresis. (g) Agarose gel electrophoreses of PCR merchandise amplified from the PD-L1 gene in HepG2 cells within the presence of T7E1 enzyme
Pharmacodynamic characterization of PTX + RNP-OCT/CS/NPs in HepG2 cells
We studied the impact of the totally different formulations on PD-L1 expression in HepG2 cells detecting by western blot. After 48 hours’ incubation, the outcomes confirmed that the expression of PD-L1 within the cells handled with PTX + RNP-NPs and PTX + RNP-OCT/CS/NPs have been considerably decreased (Fig. 4b), indicating that RNP have been efficiently transfected into HepG2 cells and successfully decreased PD-L1 gene expression. To research the therapeutic efficacy of nanoparticles in vitro, we evaluated its cytotoxicity in opposition to HepG2 cells (with excessive SSTR2 expressio [49, 50] and 293T cells (with low SSTR2 expression [51]) utilizing a typical 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl- 2 h -tetrazolium bromide (MTT) assay. When the ultimate focus of PTX was 15 µg/mL, HepG2 cells handled with PTX-Sol exhibited slight toxicity in contrast with PBS group, as a result of lipophilic PTX was arduous to be taken up by cells. The lethality of HepG2 cells within the PTX-NPs group was practically 75% at a PTX focus of 15 µg/mL, which can be associated to the buildup of positively charged nanoparticles on the floor of the cell membrane, thus inflicting injury to the cells. Against this, PTX + RNP-OCT/CS/NPs killed practically 90% of HepG2 cells on the identical focus. These outcomes point out that the synergistic impact of RNP and PTX, and focused modification of OCT and CS can considerably improve the cytotoxicity PTX + RNP-OCT/CS/NPs. (Fig. 4c). Whereas, PTX + RNP-OCT/CS/NPs didn’t have excessive toxicity on 293T cells even at a focus of 15 µg/mL, and 76% of 293T cells remained alive after remedy (Fig. 4d). It demonstrates that OCT/CS/NPs is selectively poisonous to tumor cells, however much less poisonous to regular cells. This characterization is useful for PTX + RNP-OCT/CS/NPs to enhance tumor concentrating on in vivo and cut back toxicity to regular tissues.
Wound therapeutic assay confirmed that the migration means of PTX + RNP-OCT/CS/NPs or PTX + RNP-NPs handled HepG2 cells was considerably decreased in contrast with the management group (Fig. 4e). In the meantime, PTX + RNP-OCT/CS/NPs had the strongest means to inhibit the migration of HepG2 cells. Cell migration assays demonstrated that there was evidently decreased migration functionality of HepG2 cells handled with PTX + RNP-OCT/CS/NPs, PTX + RNP-Lip2000 or PTX + RNP-NPs in comparison with PBS and PTX-Sol teams. Furthermore, PTX + RNP-OCT/CS/NPs confirmed strongest inhibition for cell migration amongst all teams. It may be defined by that OCT and CS may be focused to bind SSTR2 and CD44 receptors respectively on the floor of HepG2. These information recommend that the proposed therapies can effectively decreases the invasiveness of HepG2 tumor cells, thereby rising the efficacy of RNP.
The apoptosis of the handled cells was additionally analyzed Annexin V-FITC/PI staining and detected by move cytometry. As proven in Fig. 4f, the results of move cytometry confirmed that the majority cells have been in early-stage of apoptosis or already useless. In comparison with the PTX-Sol handled cells, PTX-NPs and PTX + RNP-NPs resulted in 1.47- and 1.50-times increased apoptosis, respectively. PTX + RNP-NPs led to increased apoptosis or necrosis charges than PTX-NPs, indicating that RNP can promote cell apoptosis. Moreover, 87.83% of cells within the PTX + RNP-OCT/CS/NPs group have been within the apoptotic or necrotic stage, which was 1.2 occasions increased than within the PTX + RNP-NPs group (73.64%), suggesting that CS and OCT contributed nanoparticle uptake and elevated apoptosis.
Taken collectively, the above outcomes confirmed the excessive effectivity of PTX + RNP-OCT/CS/NPs in delivering RNP to knock out PD-L1 in HepG2 by inhibiting the malignant organic behaviors of HepG2 cells, together with selling apoptosis and suppressing proliferation, migration and invasion of cells. Furthermore, the proposed mixture remedy technique by making use of each PTX and RNP exhibited handiest outcomes than separate administration.
Environment friendly gene modifying achieved in vitro. (a) Consultant fluorescence photographs of GFP-293T cells. (b) PD-L1 in HepG2 cells after remedy with totally different formulations. The cytotoxicity of various formulations handled HepG2 cells (c) and 293T cells (d). e) Consultant photographs of the wound therapeutic assays in HepG2 cells from 0 to 72 h. f) After 24 h of incubation with varied formulations, the apoptosis standing and total apoptosis fee have been assessed in HepG2 cells stained with FITC-Annexin V and propidium iodide (PI). All information are represented as means ± SD, n = 3
Focusing on means of OCT/CS/NPs in vivo
To research the in vivo distribution of intravenously injected OCT/CS/NPs, we employed the near-infrared fluorescent dye DiD instead of PTX and FITC-labeled bovine serum albumin (FITC-BSA) as a substitute of the Cas9 protein. We noticed a major accumulation of nanoparticles within the mouse liver, which may gain advantage the remedy of liver ailments. The fluorescence depth of DiD in tumor tissue injected with concentrating on nanoparticles (DiD + FITC-BSA-OCT/CS/NPs) was 1.5 occasions larger than that in tumor tissue receiving negatively charged nanoparticles (DiD + FITC-BSA-CS/NPs), 2.0 occasions larger than positively charged nanoparticles (DiD + FITC-BSA-NPs), and 11.7 occasions larger than the free drug resolution (DiD + FITC-BSA-Sol) (Fig. 5a and b). The change in FITC-BSA fluorescence was just like that of DiD fluorescence within the liver of mice receiving DiD + FITC-BSA-OCT/CS/NPs (Fig. S14), indicating the codelivery of small-molecule chemical medication and large-molecule protein medication in systemic circulation. The fluorescence of DiD and FITC-BSA was primarily distributed within the liver slightly than different tissues within the OCT/CS/NPs group. Notably, within the DiD + FITC-BSA-NPs group, the fluorescence was principally localized within the liver (Fig. S14). This phenomenon aligns with reported literature, specifically [52], positively charged nanoparticles usually tend to accumulate within the lungs. The disappearance of FITC-BSA within the liver could be associated to the proteolytic degradation of the protein. It means that, inside 48 h, OCT/CS/NPs mediate sustained launch and liver concentrating on of therapeutics. This may be defined by the ample hydroxyl teams in OCT/CS/NPs forming a hydrated floor that inhibits clearance by the mononuclear phagocyte system.
We additionally collected and sliced the liver tissue to search out the goal cells for the OCT/CS/NPs (Fig. 5c). By staining CD44, we discovered that the OCT/CS/NPs and CS/NPs have been colocalized with CD44. It demonstrated that OCT/CS/NPs and CS/NPs can goal hepatic cells with excessive CD44 expression, like tumor cells and hepatic stellate cell [53]. These outcomes confirmed that the supply system may extremely accrued in liver and have a sustained launch habits.
In vivo focused supply of multifunctional nanoparticles. (a) Distribution of nanoparticles in main organs at 48 h post-injection of various formulations. (b) Fluorescent quantification of liver. (c) Immunofluorescence co-localization of DiD (pink) and FITC-BSA (inexperienced) from DiD + FITC-BSA-OCT/CS/NPs and anti-CD44 antibody (purple). Cell nuclei have been stained with DAPI (blue). All information are represented as means ± SD, n = 3
Therapeutic impact of PTX + RNP-OCT/CS/NPs in tumor-bearing mice
We subsequent investigated whether or not PTX-mediated chemotherapy and CRISPR/Cas9 primarily based PD-L1 gene modifying may act synergistically to kill tumor cells. Chemotherapeutic medication have performed an necessary position in treating cancers due to their huge antitumor spectrum and direct cytotoxicity to tumor cells [54, 55]. Current research have proven that sure chemotherapeutics, corresponding to PTX, doxorubicin, and cisplatin, exhibit the power to induce immunogenic cell loss of life (ICD) in tumor cells by eliciting damage-associated molecular patterns [56]. When uncovered to those ICD inducers, tumor cells show calreticulin (CRT) on their floor and launch heightened ranges of excessive mobility group field 1 (HMGB1) [57, 58]. These phenomena improve the immunogenicity of the tumor cells and promote their phagocytosis by dendritic cells (DCs), successfully changing the dying tumor cells into “tumor vaccines” [59]. PD-L1 is a key molecule that promotes immune escape, and prevents the tumor cells from being acknowledged by the T cells [60]. Inhibiting PD-L1 expression was anticipated to reinforce tumor cell sensitivity to PTX-generated tumor vaccines and suppress tumor progress [61].
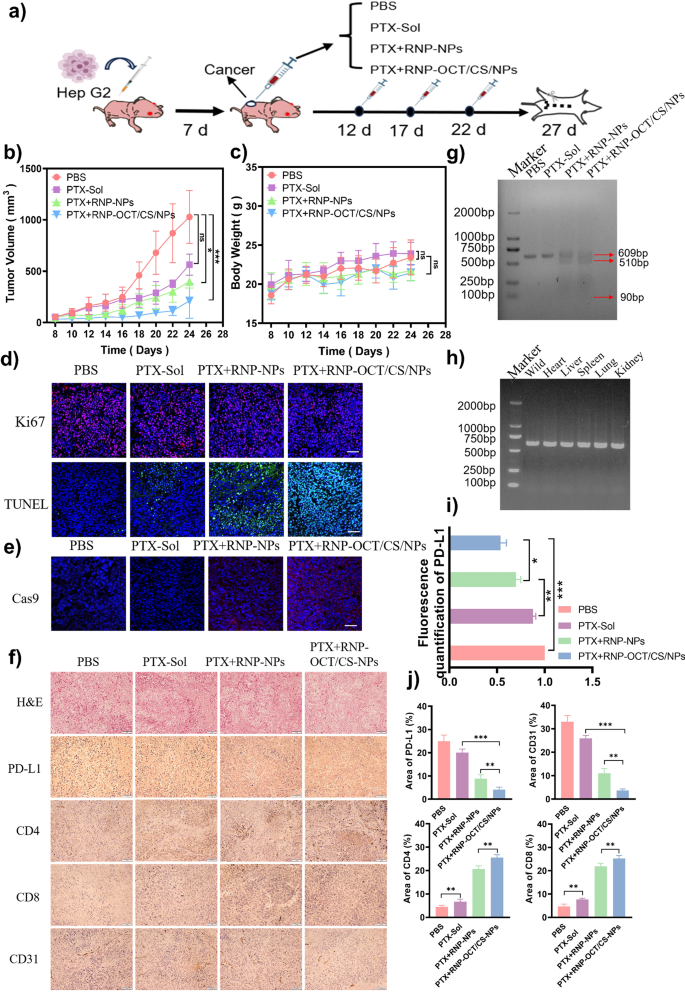
The antitumor impact of mixing PTX with CRISPR/Cas9 was confirmed in a HepG2 tumor-bearing mice mannequin. When tumor dimension reached 50 mm3, PBS, PTX-Sol, PTX + RNP-NPs, PTX + RNP-OCT/CS/NPs have been intravenously injected into mice (dose of 10 mg/kg PTX), respectively. The administration routine is proven within the Fig. 6a. The tumor quantity of the mice was recorded each different day. As proven in Fig. 6b, the tumor quantity of mouse handled with PTX + RNP-OCT/CS/NPs was a lot decrease than PTX + RNP-NPs underneath related PTX and RNP concentrations. It demonstrated that PTX + RNP-OCT/CS/NPs performed an necessary position in tumor inhibition. Goal supply of PTX and Cas9 RNP by OCT/CS/NPs improves drug accumulation on the tumor website, and PD-L1 gene modifying enhanced the tumor sensitivity to PTX.
The physique weights of mice in numerous teams didn’t change considerably through the remark interval (Fig. 6c), indicating that the PTX + RNP-OCT/CS/NPs have been secure and biocompatible. 5 days after the final injection, the mice have been euthanized and the tumors have been dissected and photographed (Fig. S15). Remoted tumor tissues obtained terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick labeling (TUNEL) assay and Ki67 immunofluorescence staining. We confirmed that remedy with PTX-Sol brought about few apoptotic cells (Fig. 6d and S16). Therapy with PTX + RNP-OCT/CS/NPs resulted in larger tumor cell apoptosis and necrosis than PTX + RNP-NPs (missing OCT and CS). The focused modification of OCT/CS/NPs primarily based on OCT and CS improves the tumor accumulation of the 2 medication and improves PTX-based chemotherapy and PD-L1 gene modifying efficacy.
Subsequent, we analyzed the power of OCT/CS/NPs for focused drug supply and gene modifying effectivity in vivo. Tumor cells receiving PTX + RNP-OCT/CS/NPs confirmed larger Cas9 expression and decrease PD-L1 protein expression than cells receiving PTX + RNP-NPs in vivo (Fig. 6e and f, and S17). Nevertheless, in tumor cells receiving PTX-Sol, Cas9 was hardly detected, and the PD-L1 protein stage was nearly the identical as within the management group. These outcomes indicated the significance of each nanoparticle encapsulation and OCT modification in Cas9 lysosomal escape (avoiding degradation) and PD-L1 gene disruption. We additionally subjected the tumor tissues to a T7EI assay. Therapies with PTX + RNP-OCT/CS/NPs and PTX + RNP-NPs resulted in 46.0% and 30.2% gene disruption effectivity, respectively (Fig. 6g). We additionally studied the off-target modifying of genes in mice handled with PTX + RNP-OCT/CS/NPs, and the outcomes are proven in Fig. 6h. There was no off-target modifying of genes within the organs of the center, liver, spleen, lungs, and kidneys. Respectively, there was a 30.2% and 46.3% discount in mRNA ranges for PTX + RNP-NPs and PTX + RNP-OCT/CS/NPs (Fig. 6i), per the outcomes of the T7EI assay.
The tumor part was stained with hematoxylin and eosin (H&E). Extra important degradation of cell membranes and necrosis have been noticed within the PTX + RNP-OCT/CS/NPs handled group in comparison with different teams, suggesting higher cytotoxicity of PTX + RNP-OCT/CS/NPs. Because the discount of PD-L1 might have an effect on the activation and differentiation of T cells [62], we carried out immunohistochemical evaluation of PD-L1, CD4, and CD8 in tumor tissues (Fig. 6f) and quantitative evaluation utilizing ImageJ (Fig. 6j). The expression of PD-L1 within the PBS-treated mice was considerably increased than within the different teams. The PTX + RNP-OCT/CS/NPs group had the bottom expression. We investigated the potential immune responses generated by our mixture remedy. Cytotoxic T lymphocytes launch perforin, granzymes, and granulysin to kill goal cells straight, whereas helper T lymphocytes regulate adaptive immunity [63]. Subsequently, we assayed the degrees of CD8+ T cells and CD4 + T cells via immunohistochemical staining in handled animals. PTX + RNP-OCT/CS/NPs remedy led to considerably increased percentages of each sorts of T cells than in management animals in tumors (Fig. 6f). Extra in depth brown areas in tumor biopsies may be seen in tumors from mice handled with PTX + RNP-OCT/CS/NPs. These outcomes recommend that PTX + RNP-OCT/CS/NPs can reverse immunosuppression within the tumor microenvironment and induce a T cell-mediated immune response resulting from PD-L1 knockout.
CD31 is a vascular endothelial cell marker that’s concerned within the formation and upkeep of recent blood vessels [64]. And in tumors, angiogenesis is essential as a result of tumors must acquire adequate vitamins and oxygen via blood vessels to assist their proliferation and survival. The expression of CD31 on tumor tissues is intently associated to tumor angiogenesis. The content material of CD31 was considerably increased within the PBS group than in different teams, and the PTX + RNP-OCT/CS/NPs group had the least variety of brown areas.
General, these outcomes show that the mixture of chemotherapy, CRISPR/Cas9 gene remedy, and focused nanoparticle design contributes to immunotherapy for HCC. PTX + RNP-OCT/CS/NPs inhibits immune evasion of tumor cells by mutating the PD-L1 gene, they usually effectively induce an antitumor immune response, together with dendritic cell maturation, manufacturing of tumor-associated antigens and cytokines, and promotion of T cells. These results might clarify how the mixture remedy can gradual the expansion and inhibit the recurrence of HCC.
In vivo experiment of antitumor exercise. (a) Schematic diagram of modeling course of and remedy strategy. (b) Tumor quantity variations over time following remedy with varied formulations. (c) Physique weight adjustments of HepG2-bearing mice handled with totally different formulations for 20 days. (d) Immunofluorescent photographs of TUNEL and Ki67. Scale bar, 100 μm. (e) Immunofluorescent photographs of Cas9. Scale bar, 100 μm. (f) H&E staining of tumor tissues and immunohistochemical evaluation of PD-L1, CD4, CD8, and CD31. Scale bar, 100 μm. (g) Frequency of indel mutation detected by T7E1 assay from tumor tissues after totally different therapies. (h) Validation of off-target gene modifying in coronary heart, liver, spleen, lungs, kidneys and different organs. (i) qRT-PCR evaluation of corresponding PD-L1 mRNA ranges. (j) Quantitative evaluation of PD-L1, CD4, CD8, CD31 ranges. All information are represented as means ± SD, n = 3
Security analysis
We carried out blood biochemical examination in mice and investigated the correlation ranges of Alkaline Phosphatase (ALP), Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), and γ-glutamyltransferase (γ-GT) (Fig. 7a). The outcomes confirmed that there was no important distinction within the ranges of those enzymes within the blood of the remedy teams, suggesting that the liver toxicity of PTX + RNP-OCT/CS/NPs was negligible. Hearts, livers, spleens, lungs, and kidneys from mice after varied therapies have been dissected and stained (Fig. 7b). H&E staining of main organs on day 20 revealed few variations between the drug-containing teams and the management group.
Security analysis. (a) Comparability of Alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyltransferase (γ-GT) between management and drug handled mice by blood biochemical examination. (b) To look at the in vivo toxicity of various therapies, coronary heart, liver, spleen, lung, and kidney of mice have been deteced utilizing H&E staining. Scale bar, 100 μm. All information are represented as means ± SD, n = 3