Preparation, purification and characterization of OMV-RBD
A schematic diagram of the OMV-RBD preparation course of is proven in Fig. 1A. OMV and RBD will be covalently linked via the amide bond fashioned between ST and SC. The SpyTag gene was modified to the upstream of the RBD gene, and RBD-ST was expressed in eukaryotic cells. The SpyCatcher gene was linked with C-terminal area of ClyA, which ensures the publicity of the SpyCatcher on the floor of OMV membrane [39] (Fig. 1B).
Preparation and characterization of OMV-RBD. A Schematic diagram of OMV-RBD preparation course of. B Building of pThio-HisA-ClyA-SC plasmid and pcDNA3.1-RBD-SpyTag plasmid. C The SDS-PAGE for screening the induction situations. The ClyA bands at 55 kDa have been totally different because of variations in IPTG induction concentrations and tradition temperatures. D The response course of and purification impact have been monitored by Western blot. The anticipated molecular weight of RBD-ST is 28 kDa. Lane 1: OMV-SC; Lane 2: RBD-ST; Lane 3: Response Product of OMV-SC and RBD-ST at a Mass Ratio of 30:1; Lane 4: Purified OMV-RBD after Ultracentrifugation. E Transmission electron microscopy of OMV-SC. F Transmission electron microscopy of OMV-SC
To be able to enhance the yield of OMV-SC, we performed a preliminary investigation of the induction situations. Orthogonal experiments have been carried out at totally different IPTG concentrations (1 mM, 0.5 mM and 0.1 mM) and totally different induction temperatures (37 ℃, 25 ℃ and 16 ℃), and the expression of ClyA-SC was examined by SDS-PAGE. As proven in Fig. 1C, ClyA-SC is the corresponding band at 55 kDa, and the expression of ClyA-SC was comparatively greater underneath the situation of 0.5 mM IPTG, in a single day tradition at 25℃, which can be comparatively milder and extra favorable for the proper folding of ClyA-SC. On this situation, roughly 1.2 mg of OMVs will be remoted on common from every 1 L of the bacterial tradition.
After the expression and quantification of RBD-ST, it could possibly be instantly reacted with OMV-SC. Based mostly on the optimized feed ratio decided within the earlier levels [37], the response was performed at a mass ratio of 30:1 between OMV-SC and RBD-ST. The protein immunoblotting technique was used to research the relative quantities of the residual RBD-ST and the ensuing OMV-RBD. To be able to take away the unreacted RBD-ST, samples have been centrifuged at 4℃, 150,000 × g for two h. This centrifugation step allowed for the entire separation of the soluble impurities from OMV-RBD. In the meantime, the content material of RBD displayed in OMV-RBD is also characterised by analyzing the grey values of the RBD-ST bands earlier than and after the response (Fig. 1D).
To make clear whether or not OMV nonetheless retained the particular morphology after the conjugation, OMV-RBD was characterised by DLS and TEM. The particle dimension distribution of OMV-RBD was usually akin to that of the pre-reaction OMV-SC (Supplementary Fig. 1). The lipid bilayer was clearly seen underneath TEM each earlier than (Fig. 1E) and after (Fig. 1F) the conjugation, exhibiting an everyday vesicle-like form, suggesting that the structural integrity of OMV was preserved after the response. As well as, no protein aggregates have been noticed underneath TEM, indicating the excessive purity of the OMV constructs. SDS-PAGE evaluation was carried out on OMV-SC and OMV-RBD (Supplementary Fig. 2), and the outcomes confirmed that the key protein bands of these samples have been extremely according to the attribute protein bands of reported E. coli-derived OMVs [40], indicating that they share related protein compositions.
OMV-RBD activated the innate immune course of in BMDCs
Bone marrow-derived dendritic cells (BMDCs), which have by no means been uncovered to antigens, have been used to guage the immunostimulatory means of OMV-RBD. The protection of OMV-RBD in vitro was confirmed by hemolysis check, CCK8 and MTT cytotoxicity check (Supplementary Fig. 3). BMDCs co-cultured with PBS, RBD or OMV-RBD have been collected for high-throughput sequencing. Principal part evaluation (PCA) was used to scale back the dimension of the information, which revealed that samples from OMV-RBD teams clustered independently from PBS or RBD teams (Fig. 2A). The gene expression ranges of samples have been calculated by the differential expression evaluation bundle DESeq2, and genes with at the very least a twofold change in expression degree and p-value lower than 0.05 have been outlined as differentially expressed genes (DEGs). In contrast with the PBS management group, the RBD-treated group confirmed minimal adjustments on the RNA degree, with solely 208 DEGs, together with 126 downregulated genes and 82 upregulated genes (Fig. 2B).
OMV-RBD activated the innate immune course of in BMDCs. A Principal part evaluation confirmed that PBS (crimson), RBD (blue) and OMV-RBD (inexperienced) handled samples in numerous teams. B Volcanic maps of DEGs within the RBD group and the PBS group (as management). These marked in crimson are up-regulated genes (LFC > 1, P < 0.05), blue marked for down-regulated genes (LFC < −1, P < 0.05). The names of genes with vital and consultant adjustments have been highlighted. C Volcanic maps of differentially expressed genes (DEGs) within the OMV-RBD group and the PBS group (as management). D Volcanic maps of DEGs in within the OMV-RBD group and the RBD group (as management). E Warmth maps of unsupervised cluster DEGs in samples. F Genes up-regulated within the OMV-RBD group in comparison with the PBS (high) or RBD (backside) teams have been clustered and annotated utilizing Metscape, respectively, and visualized utilizing bar graphs after collapsing redundant tags
As well as, cluster evaluation outcomes indicated that the DEGs within the PBS and RBD-treated teams have been extremely related (Fig. 2E). Notably, after RBD-OMV therapy, there have been 4816 differentially expressed genes in comparison with the PBS therapy group, with 2001 genes upregulated and 2815 genes downregulated (Fig. 2C). Equally, in comparison with the RBD-treated group, there have been 4803 differentially expressed genes, together with 1974 genes downregulated and 2829 genes upregulated (Fig. 2D). The above differential gene units have been clustered and annotated by Metascape, respectively, and the redundant tags have been additional collapsed. The outcomes confirmed that genes associated to the immune activation course of have been considerably upregulated after RBD-OMV therapy (Fig. 2E–F). In distinction, the upregulated gene units after RBD therapy alone weren’t enriched for immune-related organic processes and signaling pathways, probably as a result of comparatively low immunogenicity of RBD alone and the shortcoming to additional activate BMDCs (Supplementary Fig. 4).
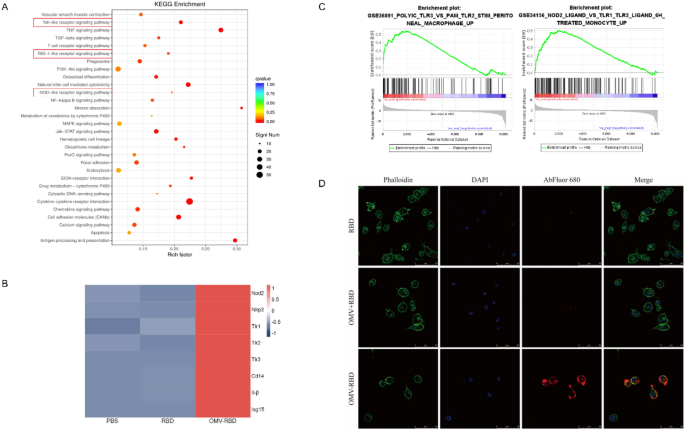
OMV-RBD activated NOD2 and TLR3 on the floor of BMDCs
Correct antigen recognition and environment friendly phagocytosis by dendritic cells (DCs) is step one in constructive immune activation. A sequence of sample recognition receptors (PRRs), corresponding to Toll-like receptors (TLRs), Nod-like receptors (NLRs), retinoic acid-inducing gene (RIG) I-like receptors (RLRs), and extra, act because the eyes of DCs to acknowledge pathogen-associated molecular patterns and additional facilitate antigen internalization. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment evaluation of the up-regulated gene units after OMV-RBD therapy confirmed that three PRRs, together with TLRs, NLRs, and RLRs, have been activated (Fig. 3A). To keep away from the interference of crosstalk genes between signaling pathways, the relative expression ranges of core genes within the three PRR pathways have been in contrast and visualized by warmth maps. In short, the expression ranges of key elements within the NLR pathway (Nod2 and Nlrp3), the TLR pathway (Tlr1, Tlr2, Tlr3, Cd14, Il-1β) and the RLR pathway (Isg15) have been considerably elevated following therapy with OMV-RBD (Fig. 3B).
OMV-RBD activated NOD2 and TLR3 on the floor of BMDCs. A Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway evaluation of up-regulated genes in OMV-RBD group vs. PBS group (as management). B Heatmap of the core genes within the chosen pathway. Gene expression was measured by Transcripts Per Million (TPM) and represented by colour. C Gene expression profile evaluation by gene set enrichment evaluation (GSEA) revealed vital enrichment of TLR3 and NOD2 signaling pathways associated gene options of OMV-RBD up-regulated genes (P < 0.05). Y-axes point out enrichment scores (high) and ranked listing metric (backside). X-axis bars signify particular person genes of the indicated gene units. D OMV-RBD can promote recognition and phagocytosis of dendritic cells. The confocal microscope pictures of BMDCs incubated with RBD, OMV + RBD and OMV-RBD respectively. The cytoskeleton was stained with TRITC phalloidin, and the nucleus was displayed by binding DAPI. RBD was labeled with Abfluor™ 680
Particularly, the outcomes from “GSE36891 POLYIC TLR3 VS PAM TLR2 STIM PERITONEAL MACROPHAGE UP” and “GSE34156 NOD2 LIGAND VS TLR1 TLR2 LIGAND 6H TREATED MONOCYTE UP” indicated the activation of TLR3 and NOD2 (Fig. 3C). It’s well-known that these signaling pathways play an vital position in antiviral immunity, suggesting that OMV-RBD is extremely advantageous as an antiviral vaccine. Abfluor™ 680-labeled RBD, OMV + RBD and OMV-RBD have been incubated with BMDCs for twenty-four h, and the antigen uptake of BMDCs was noticed underneath a confocal microscope to analyze whether or not PRRs activation might promote the correct recognition and environment friendly absorption of antigens. Apparently, solely the coupling (OMV-RBD) however not the bodily mixing (OMV + RBD) might promote antigen uptake by BMDCs (Fig. 3 D).
OMV-RBD confered the flexibility of BMDCs to provoke adaptive immune responses
The outcomes of Gene Set Enrichment Evaluation (GSEA) demonstrated that DCs co-cultured with OMV-RBD exhibit substantial alterations in each phenotype and performance upon antigen recognition and phagocytosis through PRRs, finally transitioning to a state of mobile maturation (Fig. 4A). Immature DCs have robust migration means. On the identical time, mature DCs can successfully activate naive T cells, that are the central hyperlink of initiating, regulating and sustaining immune response.
OMV-RBD confered the flexibility of BMDCs to provoke adaptive immune responses. A Enrichment plots of Lindstedt DC maturation gene units exhibiting vital upregulation in OMV-RBD handled BMDCs. Consultant plots C and statistical plots B confirmed the floor expression of CD40, CD80 and CD86 in CD11c + cell inhabitants after co-incubation of BMDCs with RBD, LTK63 + RBD, OMV + RBD and OMV-RBD respectively. D The cytokines within the supernatant of BMDCs tradition have been detected by ELISA. Knowledge are expressed as imply ± S.D., n = 3. (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
Mature DCs are characterised by gradual lack of phagocytic means, elevated expression of co-stimulatory molecules on its floor and secretion of a wide range of cytokines. The expression ranges of three co-stimulatory molecules (CD40, CD80, and CD86) have been quantified utilizing circulation cytometry subsequent to the co-incubation of BMDCs with RBD alone, LTK63 + RBD, OMV + RBD or OMV-RBD (Fig. 4B–C). The classical mucosal adjuvant LTK63, an E. coli enterotoxin mutant, was used as a constructive management. In contrast with the RBD group, there was no vital change within the proportion of CD40-positive cells within the LTK63 + RBD, OMV + RBD or OMV-RBD teams. Nonetheless, the proportion of CD80 and CD86 constructive cells was considerably elevated (The gating technique was proven in Supplementary Fig. 5). These outcomes indicated that the potent means of OMV to stimulate DC maturation was efficiently retained within the built-in vaccine obtained by coupling OMV with antigen.
Alternatively, immune-related cytokines within the tradition supernatants of BMDCs have been detected by ELISA. It’s well-known that IL-1β, IL-6, IL-12, and TNF-α are key pro-inflammatory cytokines that play vital roles in each the innate immune response and the adaptive response. In distinction, IL-10, as a pleiotropic cytokine with potent anti-inflammatory properties, is ready to inhibit the antigen presentation means of dendritic cells at excessive concentrations, leading to inefficient T-cell activation. In our research, the degrees of pro-inflammatory cytokines (IL-1β, IL-6, IL-12, and TNF-α) within the supernatants of OMV + RBD and OMV-RBD co-cultured with BMDCs have been considerably elevated. In distinction, the degrees of anti-inflammatory cytokines (IL-10) have been considerably decreased, indicating that each can successfully promote the maturation of dendritic cells (Fig. 4D).
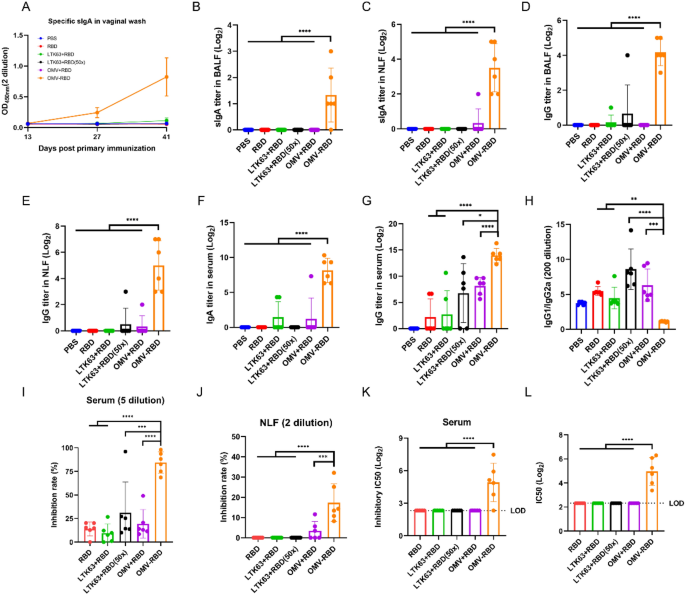
OMV-RBD vaccine enhanced mucosal and systemic antibody ranges with potent neutralizing capability through intranasal immunization
Initially, the protection of the OMV-RBD vaccine in vivo was confirmed by gathering serum to detect liver and kidney perform and gathering the guts, liver, spleen, lung and kidney of mice to make pathological sections (Supplementary Fig. 6–7). Particularly, after mice have been immunized 3 times, serum biochemical indices have been assessed and no vital variations have been discovered within the concentrations of albumin (Alb), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (Cr), alkaline phosphatase (ALP), uric acid (UA), and direct bilirubin (DBIL). These outcomes indicated that no obvious liver, kidney, or different organ dysfunction was attributable to the immunization protocol. Moreover, no noticeable indicators of irritation have been exhibited by the pathological sections of the collected organs, additional supporting the protection of the immunization process. To research the dynamics of RBD-specific mucosal antibody ranges in relation to the frequency of intranasal immunization, the degrees of sIgA antibody within the vaginal wash have been monitored (Fig. 5A). In contrast with different teams, mice immunized with the OMV-RBD vaccine exhibited an preliminary elevation in sIgA ranges subsequent to the second immunization, which was additional augmented by a considerable improve following the third immunization, suggesting that the administration of three doses of the OMV-RBD vaccine is important for attaining an optimum immune response.
Humoral response of intranasally immunized mice. A The degrees of mouse sIgA antibody modified in vaginal wash post-immunization. B Particular sIgA titers in BALF. C Particular sIgA titers in NLF. D Particular IgG titers in BALF. E Particular IgG titers in NLF. F Particular sIgA titers in serum. G Particular IgG titers in serum. H The ratio of IgG1/IgG2a in serum. I hACE2 aggressive inhibition price of serum; (J) hACE2 aggressive inhibition price of NLF; Ok IC50 of hACE2 aggressive inhibition in serum. L Serum pseudovirus neutralizing antibody ranges. Knowledge are expressed as imply ± S.D., n = 6. (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
By assessing the sIgA antibody titers in BALF and NLF 14 days post-final immunization, RBD-specific sIgA titers on the respiratory mucosal websites have been considerably greater within the OMV-RBD vaccine group in comparison with the opposite teams (Fig. 5B–C). Notably, the bodily combined RBD with OMV or LTK63 didn’t elicit the particular sIgA response, even when the dose of RBD was elevated by 50-fold when mixed with LTK63. In keeping with the observations concerning the sIgA response, immunization with OMV-RBD considerably elevated the degrees of particular IgG antibodies in BALF (Fig. 5D) and NLF (Fig. 5E). As well as, the serum RBD-specific IgA (Fig. 5F) and IgG (Fig. 5G) titers of mice immunized with OMV-RBD vaccine have been additionally considerably greater than these of free RBD or mixture with OMV or LTK63, underscoring the crucial position of the OMV-RBD conjugate in stimulating sturdy humoral responses. The bottom IgG1/IgG2a ratio was noticed within the OMV-RBD vaccine group (Fig. 5H), indicating that it was simpler in selling a Th1-biased response.
To additional characterize the neutralization efficacy of the antibodies, serum, BALF, and nasal wash have been collected 14 days post-final immunization. OMV-RBD group exhibited a marked enhancement within the capability of each serum (Fig. 5I) and NLF (Fig. 5J) to inhibit RBD-ACE2 interplay, a crucial step for viral entry into host cells. The pseudovirus neutralization assay additionally confirmed that the OMV-RBD vaccine induced the very best ranges of neutralizing antibody titers (Fig. 5Ok-L), suggesting that intranasal immunization with the OMV-RBD vaccine might considerably improve the neutralizing means of antibodies.
OMV-RBD vaccine promoted Th1-biased responses through intranasal immunization
The vaccine-induced T-cell response performs an important position within the COVID-19 vaccine protecting efficacy. To evaluate the extent of mobile immune response induced by OMV-RBD vaccine, ELISpot was used to detect the variety of IFN-γ secreting cells in splenic and pulmonary lymphocytes upon stimulation with RBD peptide pool (18-mers overlapping by 9 aa individually). OMV-RBD was able to markedly augmenting the pulmonary T-cell response, as evidenced by a notable improve within the frequency of IFN-γ producing cells (Fig. 6A–B). Moreover, in splenic lymphocytes, the OMV-RBD group exhibited a discernible upward pattern within the variety of IFN-γ secreting cells, as in comparison with the opposite teams (Fig. 6C–D). Though this pattern didn’t attain statistical significance, it gives preliminary indications of a possible enhancement in splenic T cell activation.
Mobile response of intranasally immunized mice. A The variety of RBD-specific IFN-γ secretory cells in 106 pulmonary lymphocytes. B Consultant ELISpot outcomes of pulmonary lymphocytes. C The variety of RBD-specific IFN-γ secretory cells in 106 spleen lymphocytes. D Consultant ELISpot outcomes of spleen lymphocytes. E The focus of IFN-γ. F The focus of IL-2. G The focus of IL-4. H The focus of IL-17. Knowledge are expressed as imply ± S.D., n = 6. (*P < 0.05, **P < 0.01, ***P < 0.001)
In parallel to the ELISpot evaluation, the concentrations of cytokines together with IFN-γ, IL-4, IL-17 and IL-2 within the supernatants of stimulated mouse splenic lymphocytes have been measured by ELISA. Whereas the degrees of IFN-γ (Fig. 6E), IL-4 (Fig. 6F), and IL-17 (Fig. 6G) induced by each OMV-RBD and OMV + RBD didn’t considerably differ from these noticed within the RBD group alone, a notable upward pattern was noticed for these cytokines. In the meantime, IL-2 ranges induced by OMV-RBD have been considerably elevated (Fig. 6H). IL-2 is a key cytokine concerned within the proliferation and differentiation of T cells, notably Th1 cells, suggesting that intranasal immunization with OMV-RBD successfully promotes a Th1-biased immune response.
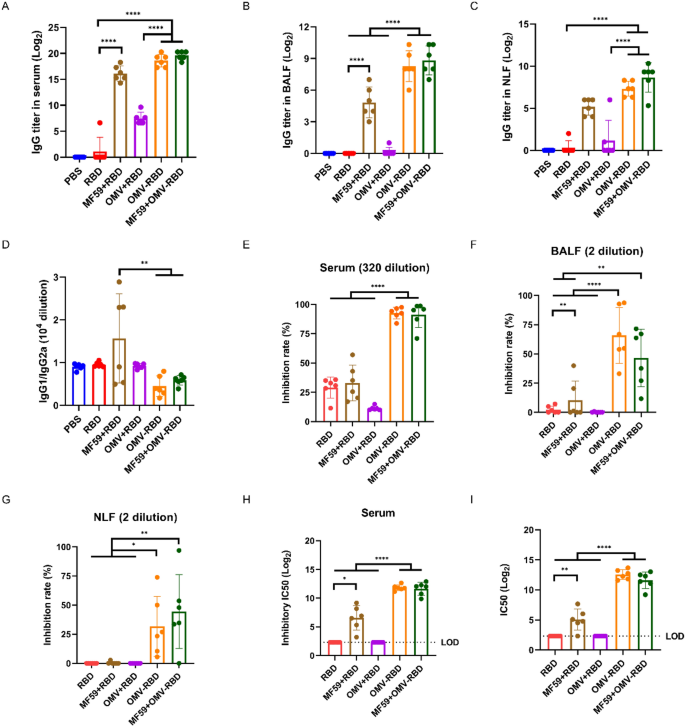
OMV-RBD vaccine outperformed MF59-adjuvanted RBD in inducing neutralizing antibodies through intramuscular immunization
According to our intranasal immunization findings, intramuscular administration of OMV-RBD considerably elicited greater IgG antibody titers in serum (Fig. 7A), BALF (Fig. 7B), and NLF (Fig. 7C) in comparison with RBD and OMV + RBD teams. When in comparison with MF59 + RBD, the rise in IgG titers with OMV-RBD was evident however didn’t attain statistical significance, indicating a comparable immunogenicity. Notably, whereas the MF59 adjuvant considerably enhanced IgG titers when mixed with RBD, its addition to OMV-RBD didn’t additional elevate antibody ranges, suggesting that OMV-RBD alone, when administered intramuscularly, is able to attaining sturdy antibody titers with out the necessity for extra adjuvants. Moreover, IgG subclass ratios revealed that the MF59 + RBD mixture skewed the immune response in the direction of a Th2 bias, as evidenced by elevated IgG1/IgG2a ratios (Fig. 7D). In distinction, OMV-RBD, each intranasally and intramuscularly, maintained a Th1 bias, as indicated by the comparatively decrease IgG1/IgG2a values. This statement underscores the flexibility of OMV-RBD to constantly elicit a Th1-dominant immune response, whatever the route of administration, which is essential for efficient antiviral immunity.
Humoral response of intramuscularly immunized mice. A Particular IgG titers in serum. B Particular IgG titers in BALF. C Particular IgG titers in NLF. D The ratio of IgG1/IgG2a in serum. E hACE2 aggressive inhibition price of serum; F hACE2 aggressive inhibition price of BALF; G hACE2 aggressive inhibition price of NLF. H IC50 of hACE2 aggressive inhibition in serum. (I) Serum pseudovirus neutralizing antibody ranges. Knowledge are expressed as imply ± S.D., n = 6. (*P < 0.05, **P < 0.01, ****P < 0.0001)
The neutralizing antibodies of intramuscularly immunized mice have been examined by hACE2 aggressive inhibition assay and pseudovirus neutralization experiment, and it was discovered that the inhibition price of antibodies in serum (Fig. 7E), BALF (Fig. 7F), and NLF (Fig. 7G) induced by OMV-RBD was considerably greater than these of the opposite vaccine formulations. The serum IC50 (LOD = 2.32) was notably elevated for each the inhibition of RBD-ACE2 binding (Fig. 7H) and pseudovirus neutralization (Fig. 7I). Regardless of the enhancement of RBD immunogenicity by the MF59 adjuvant, its inclusion didn’t additional amplify the technology of upper titers of neutralizing antibodies induced by the OMV-RBD vaccine.
OMV-RBD vaccine elicited potent Th1 and Th17 responses through intramuscular immunization
To judge the mobile immune response induced by intramuscular injection of OMV-RBD vaccine, ELISpot assay was used to quantify the variety of IFN-γ-secreting splenic lymphocytes. As proven in Fig. 8A–B, a marked improve within the variety of IFN-γ-secreting cells was noticed within the OMV-RBD group, and the inclusion of MF59 adjuvant didn’t additional increase this response, indicating that the OMV-RBD vaccine alone is adequate to elicit sturdy mobile immunity.
Mobile response of intramuscularly immunized mice. A The variety of RBD-specific IFN-γ secretory cells in 106 spleen lymphocytes. B Consultant ELISpot outcomes of spleen lymphocytes. C The focus of IFN-γ. D The focus of IL-2. E The focus of IL-4. F The focus of IL-17. Knowledge are expressed as imply ± S.D., n = 6. (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
To be able to additional examine the traits of the T cell response, the concentrations of IFN-γ (Fig. 8C), IL-4 (Fig. 8D), IL-17 (Fig. 8E) and IL-2 (Fig. 8F) within the supernatants of the splenic lymphocytes have been measured by ELISA. The degrees of IFN-γ within the OMV-RBD group have been considerably elevated in comparison with these in the RBD and OMV + RBD teams, according to the ELISpot findings. Furthermore, the focus of IL-2 within the OMV-RBD group additionally had a bent to extend in contrast with that of RBD, additional supporting the notion that the OMV-RBD vaccine promoted a Th1-biased response. In the meantime, the OMV-RBD vaccine additionally induced a major improve in IL-17 secretion, highlighting the twin Th1 and Th17 mobile immune response elicited by this vaccine.