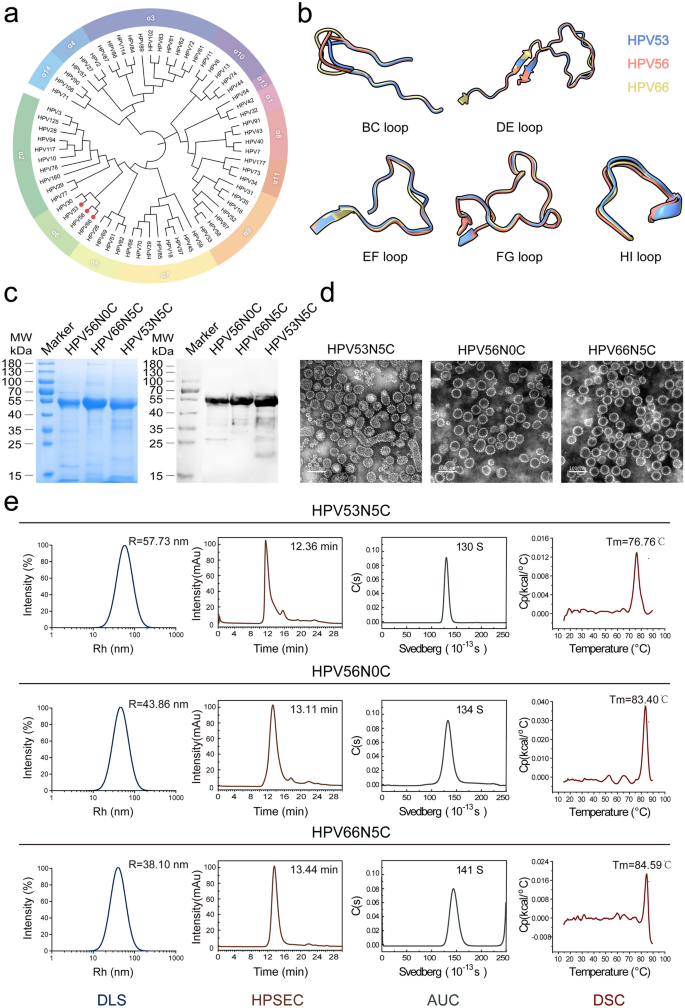
Phylogenetic classification and conservation evaluation of HPV L1 and its floor loops
We obtained 231 consultant HPV sorts L1 sequences from the Worldwide Human Papillomavirus Reference Middle [33]. In keeping with the IARC carcinogenicity classification, all recognized carcinogenic or presumably carcinogenic HPV sorts belong to the Alpha genus [4]. Consequently, we analyzed the evolutionary relationships of L1 protein sequences throughout all 65 reported HPV sorts inside the Alpha genus, focusing particularly on the prevalence of 25 probably carcinogenic HPV sorts and the low-risk sorts HPV6 and HPV11 amongst girls with invasive cervical most cancers in China and globally [2, 56]. As proven in Fig. 1a, HPV53, HPV56, and HPV66 belong to the Alpha 6 group, and their L1 proteins are evolutionarily nearer to one another than to different high-risk or low-risk sorts recognized by the IARC.
Evolutionary relationships and biophysical characterization of HPV53, HPV56, and HPV66 L1 protein constructs. a Phylogenetic evaluation of 65 alpha-genus HPV sorts based mostly on L1 protein sequences, with HPV53, HPV56, and HPV66 highlighted inside the α6 group, indicating their shut evolutionary relationship inside this clade. b Comparative structural evaluation of the BC, DE, EF, FG, and HI floor loops within the L1 proteins of HPV53 (blue), HPV56 (crimson), and HPV66 (yellow), as predicted by the AlphaFold3 server. c SDS-PAGE and WB evaluation of purified N-terminally truncated WT L1 proteins. Western blot evaluation was carried out underneath decreasing situations utilizing SDS-PAGE, with the broad-spectrum linear monoclonal antibody 4B3 for detection. d TEM pictures of the VLPs, with a scale bar representing 100 nm. e Biophysical characterization of the WT VLPs, together with DLS for particle dimension distribution, HPSEC for elution profiles, AUC for sedimentation coefficients, and DSC for thermal stability profiles, offering complete insights into the structural integrity and stability of the VLPs
Utilizing Clustal Omega software program, we assessed the amino acid sequence homology of HPV53, HPV56, and HPV66 L1 proteins, revealing a considerable common homology of 75.70%. Notably, HPV56 and HPV66 exhibited a excessive sequence similarity of 87.87%, whereas HPV53 confirmed barely decrease homology with the opposite two, at 79.13% with HPV56 and 78.73% with HPV66 (Supplementary Fig. 1). To evaluate conservation inside 5 key surface-exposed loops (BC, DE, EF, FG, and HI) of the HPV L1 protein, aligned sequences for every loop have been uploaded to WebLogo (model 3.7.12) to generate conservation profiles. The evaluation indicated that whereas the loop areas of 65 Alpha-genus HPV L1 proteins have been typically much less conserved (Supplementary Fig. 2), the loop constructions of HPV53, HPV56, and HPV66—every belonging to the Alpha 6 group—displayed higher similarity. As proven in Fig. 1b, structural predictions generated by AlphaFold3 reveal substantial overlap within the FG, and HI loops of HPV53, HPV56, and HPV66, suggesting these areas as promising targets for vaccine design on account of their immunogenic potential.
Expression, purification, and characterization of HPV53, 56 and 66 WT VLPs
Gene encoding for the HPV53, HPV56 and HPV66 L1 proteins have been ready as constructs with numerous N-terminal truncations (5-, 10-, 15-aa), and cloned into the pTO-T7 vector for protein expression in E. coli system. This led to the purification of extremely pure HPV53, HPV56, and HPV66 L1 proteins, every with a molecular weight of roughly 55 kDa. These purified proteins have been remoted via a mix of cation change column chromatography and Superdex 200 column chromatography, as illustrated in Fig. 1c. Subsequent in vitro assays facilitated the self-assembly of HPV VLPs. To comprehensively characterize the bodily and chemical attributes of those VLPs, an array of analytical methods was deployed, together with TEM, HPSEC, AUC, DLS, and DSC.
TEM evaluation offered important insights into the VLPs’ morphological traits (Fig. 1d). Majorly, the HPV53 VLPs displayed a mixture of hole spherules, together with some irregularly formed particles, whereas HPV56 and HPV66 VLPs introduced a homogeneous inhabitants of spherules in dimension and form. DLS knowledge highlighted variations in particle dimension among the many VLPs, with imply radii of 57.73 nm for HPV53, 43.86 nm for HPV56, and 38.10 nm for HPV66 VLPs. This pattern was mirrored in HPSEC outcomes, the place the retention instances inversely correlated with particle dimension—12.36 min for HPV53, 13.11 min for HPV56, and 13.44 min for HPV66. Sedimentation coefficients derived from AUC evaluation have been 130 S for HPV53, 134 S for HPV56, and 141 S for HPV66 VLPs, reinforcing the excessive purity and uniformity of the assembled VLPs. Considerably, thermal stability assessments revealed Tm values of 76.76 °C for HPV53, 83.40 °C for HPV56, and 84.59 °C for HPV66 VLPs, indicating notable thermal stability throughout all three sorts (Fig. 1e).
WT HPV53, 56, and 66 VLPs exhibit sturdy type-specific immunogenicity with restricted cross-neutralization reactivity
To analyze the immunogenicity and cross-reactivity of WT HPV53, HPV56, and HPV66 VLPs, SPF BALB/c feminine mice aged 6 weeks have been randomly assigned into teams (n = 5) and have been subsequently immunized through intraperitoneal injection, using aluminum adjuvant at doses of 5.0 μg, 1.0 μg, and 0.2 μg per mouse. The immunization routine spanned 0, 2, and 4 weeks, with orbital venous blood assortment performed on the 6-week mark.
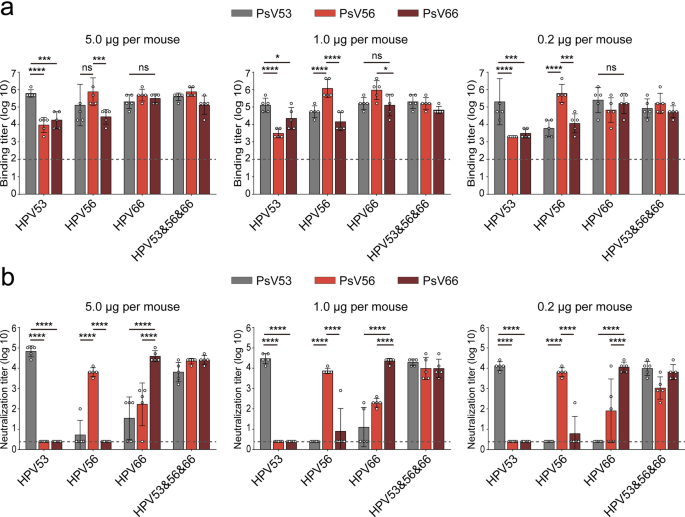
Using ELISA, we quantified the elicited titer ranges of type-specific antibodies and people able to cross-type binding towards HPV53, HPV56, and HPV66 WT VLPs, along with a mixed VLP preparation. Remarkably, as illustrated in Fig. 2a, the induced type-specific binding antibody titers for HPV53, HPV56, and HPV66 spanned from 10^5 to 10^7 throughout the administered dosages, indicating potent immunogenicity. Furthermore, these antibodies demonstrated important ranges of cross-reactivity; particularly notable was the similarity in titer ranges towards HPV53 and HPV56 when induced by HPV66, approaching the autologous titer towards HPV66 itself (p > 0.05 for each the 5.0 and 0.2 μg teams).
Vaccination with HPV53, HPV56, and HPV66 WT VLPs induces particular and cross-reactive binding and neutralizing antibodies. a Quantification of binding antibody titers in vaccinated BALB/c mice utilizing ELISA. b Measurement of neutralizing antibody titers in serum samples via a PBNA. BALB/c mice (n = 5) have been intraperitoneally inoculated with excessive (5.0 μg/dose), center (1.0 μg/dose), or low (0.2 μg/dose) doses of WT HPV53, HPV56, and HPV66 VLPs at weeks 0, 2, and 4. Binding and neutralization antibody ranges have been assessed at week 6 after the preliminary vaccination. All knowledge have been subjected to two-way ANOVA and are introduced as imply ± normal deviation (SD); *P < 0.05, **P < 0.01; ***P < 0.001; ****P < 0.0001. The error bars signify the SD, and symbols denote particular person mice
Additional evaluation of the immune responses was performed through a PBNA, assessing each type-specific and cross-type neutralizing antibody titers towards HPV53, HPV56, and HPV66. Offered in Fig. 2b, the findings revealed that every VLP kind efficiently elicited sturdy type-specific neutralizing antibodies, with titers starting from 10^4 to 10^5 among the many dosing teams. Nonetheless, the induction of cross-neutralizing antibodies between the HPV sorts was extra nuanced. The evaluation indicated a variable efficacy in cross-neutralization, with typically increased antibody titers noticed amongst genetically nearer HPV sorts. Particularly, HPV66 elicited average cross-neutralizing antibody titers towards HPV56, but it was notably much less efficient in producing cross-neutralizing responses in direction of the extra genetically distant HPV53 (p < 0.0001). This discovering is corroborated by the outcomes of the ED50 assay, the place the ED50 metric denotes the minimal efficient dose required to attain seroconversion in 50% of the topics inside a specified experimental group. As depicted in Desk 1 and Supplementary Desk 1, for his or her respective variants, HPV53, HPV56, and HPV66 demonstrated ED50 values of 0.004 μg, 0.019 μg, and 0.043 μg, respectively. Notably, whereas HPV53 and HPV56 have been ineffective at inducing seroconversion in not less than half of the mice when trying cross-protection towards different sorts, HPV66 uniquely succeeded in facilitating seropositive conversion in 5 mice towards the HPV56.
Impression of spine kind choice on meeting and particle uniformity in HPV53/56/66 cross-type immunogen designed
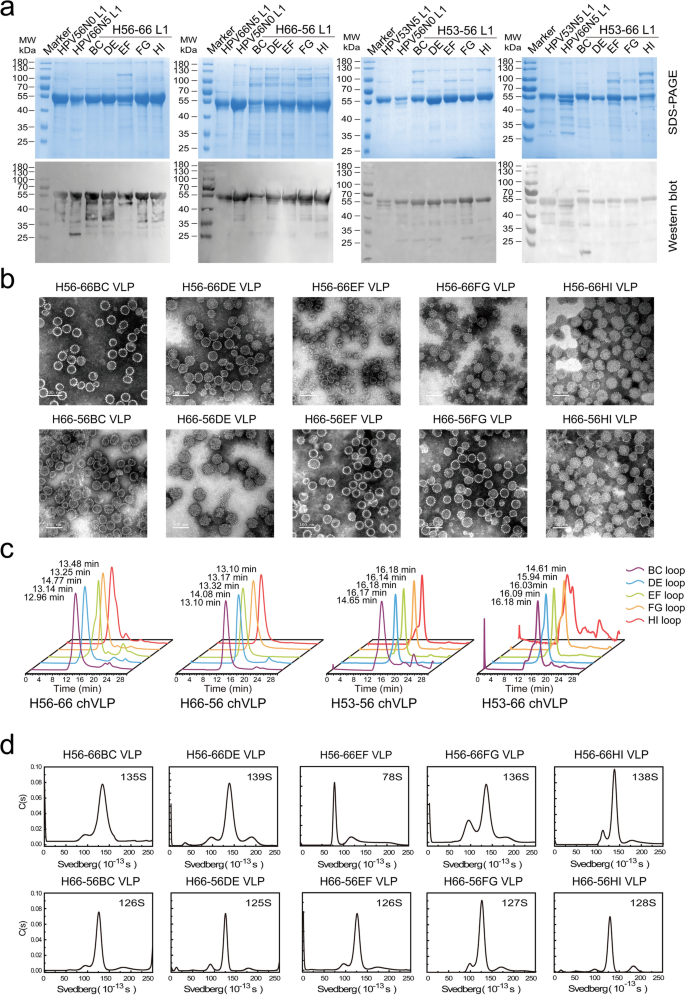
The development of triple kind chimeric molecules throughout HPV 53, HPV56, and HPV66 represents a formidable endeavor in our research. Preliminary estimates counsel that via floor loop swapping, not less than 60 distinctive tripartite chimeric VLP configurations may theoretically be realized. Nonetheless, the practicality of such a process is daunting, underscored by the immense workload concerned. A extra streamlined method, favoring the preliminary mixture of two HPV sorts earlier than integrating the third, stands to considerably reduce the verification workload of chimeric constructs. The research systematically examines the affect of spine sorts picks on the meeting effectivity and immunogenicity of chimeric molecules, a facet hitherto unexplored in earlier analysis. Specializing in HPV53, HPV56, and HPV66 as spine sorts, the investigation meticulously compares the consequences of various immunogenic epitope reshaping methods. Particularly, ten chimeric proteins using HPV56 and HPV66 as spine sorts (outlined as H56-66 BC/DE/EF/FG/HI and H66-56 BC/DE/EF/FG/HI, respectively) have been designed. Parallel efforts noticed using HPV53 as a spine, with homologous loop areas successively swapped with these from HPV56 and HPV66, leading to an extra ten chimeric constructs (notably, H53-56 BC/DE/EF/FG/HI and H53-66 BC/DE/EF/FG/HI). Expression of all double kind chimeric proteins was achieved in E. coli. Subsequently, the engineered L1 proteins underwent purification to excessive purity through a meticulously optimized two-step column chromatography course of (illustrated in Fig. 3a) earlier than being subjected to in vitro self-assembly.
Complete evaluation of the double-type chimeric VLPs intertype HPV53/56/66. Each the WT and chimeric L1 proteins have been subjected to decreasing SDS-PAGE (a) and western blotting (b) with a wide-spectrum linear mAb 4B3. TEM imaging (scale bar: 100 nm) (c) and HPSEC profiles (d) reveal the morphology and dimension distribution of H56-66, H66-56, H53-56, and H53-66 chimeric VLPs. (e) AUC profiles spotlight the sedimentation coefficients, aiding in understanding the complicated dynamics of H56-66 and H66-56 VLPs in resolution. These analyses present insights into the bodily and chemical properties of the double-type chimeric VLPs, as in comparison with the WT VLPs
Analytical scrutiny through TEM (demonstrated in Fig. 3b and Supplementary Fig. 3), and HPSEC (Fig. 3c), revealed distinctive particulate attributes—particularly, morphology and uniformity—throughout the double-type chimeric VLPs contingent on the kind of spine employed. It was noticed that chimeric proteins with HPV56 and HPV66 serving because the spine have been predominantly competent in assembling in vitro into VLPs bearing shut resemblance to the wild-type, entity, characterised by HPSEC column retention instances spanning 12–14 min. In distinction, chimeric VLPs with HPV53 because the foundational spine exhibited appreciable heterogeneity in dimension and demonstrably inferior particulate morphology, evidenced by protracted HPSEC column retention instances of 14–16 min. Additional AUC of the ten most proficiently assembled chimeric VLPs that includes HPV56 and HPV66 as spine sorts (H56-66 and H66-56) delineated sedimentation coefficients starting from 125–140 S, analogous to these noticed within the wild-type, with the singular exception of H56-66EF which manifested indicators of suboptimal meeting. These compellingly counsel that chimeric constructs between the genetically nearer HPV56 and HPV66 facilitate a preservation of physicochemical traits akin to the wild-type, portraying HPV53—owing to its marginal genetic dissimilarity—as a much less favorable candidate for the skeletal spine in chimeric molecule design.
Spine sorts and their distinctive influence on cross-type immunogenicity in chimeric VLPs
To evaluate the immunogenicity and potential cross-neutralization capabilities of chimeric VLPs based mostly on totally different spine sorts, we formulated all 20 double-type chimeric VLPs with aluminum adjuvant and administered them to feminine BALB/c mice through three intraperitoneal injections. The elicited type-specific and cross-type neutralizing antibody titers have been assiduously quantified utilizing the PBNA, yielding insightful knowledge into the molecular intricacies of VLP-induced immunity.
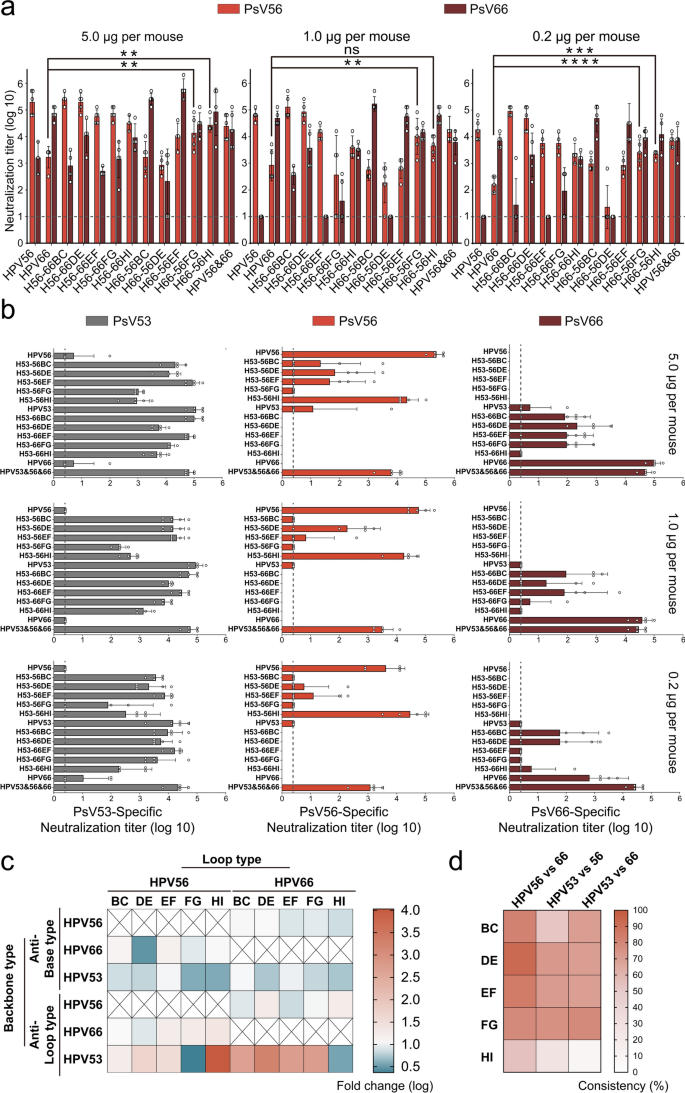
As illustrated in Fig. 4a, the investigation into 5 chimeric VLPs using HPV56 as their foundational spine resulted in diversified neutralizing antibody titers towards HPV56. Notably, the swapping of EF, FG, and HI loops with these from HPV66 (H56-66EF/FG/HI) led to a delicate discount in titers in comparison with the WT HPV56, particularly pronounced with the HI loop alternative. Regardless of these variations, the neutralization efficiency towards HPV56 sustained a excessive and dose-responsive tier, implying a pivotal function of the EF, FG, and HI loops in steering type-specific neutralizing responses in direction of HPV56. Moreover, the comparative evaluation of neutralizing antibody titers towards HPV66, induced by H56-66 chimeric VLPs, underscored the efficacy of DE and HI loop substitutions, with H56-66DE and H56-66HI eliciting titers peaking at 10^4 towards HPV66.
Cross-immunogenicity induction by double-type chimeric VLPs based mostly on various spine kind. Evaluation of the immunogenic response induced by HPV56/66 (a), H53-56 and H53-66 (b) chimeric VLPs in BALB/c mice subjected to a spread of immunization dosages. Teams of 5 BALB/c mice acquired intraperitoneal injections of both excessive (5.0 μg/dose), medium (1.0 μg/dose), or low (0.2 μg/dose) concentrations of WT HPV56, HPV66, and HPV56/66 chimeric VLPs on a schedule at weeks 0, 2, and 4. The elicitation of neutralizing antibodies was quantitatively assessed six weeks following the preliminary immunization, using neutralization assays with the assay’s sensitivity threshold delineated by a dotted line. All knowledge have been subjected to one-way ANOVA and are introduced as imply ± normal deviation (SD); *P < 0.05, **P < 0.01; ***P < 0.001; ****P < 0.0001. The error bars signify the SD, and symbols denote particular person mice. c Heatmap illustration of logarithmic fold modifications in neutralizing antibody titers focusing on each spine and loop sorts, elicited by high-dose administrations of double-type chimeric VLPs alongside these induced by WT VLP. (d) Heatmap illustrating the sequence consistency throughout homologous loop areas of HPV56, HPV66, and HPV53. Darker shades point out increased ranges of consistency
Parallel outcomes have been noticed with chimeric VLPs having HPV66 because the spine (H66-56), the place the substitution of the DE loop considerably impacted the immunogenicity towards HPV66, pointing to the DE loop’s function as a potent neutralization epitope of HPV66. The strategic reconfiguration of HPV56’s EF, FG, and HI loops onto the HPV66 spine markedly elevated type-specific neutralizing antibody ranges towards HPV56 over these induced by WT HPV66 VLPs, particularly pronounced with the HI loop alternative. Particularly, the H66-56HI chimera invoking a noteworthy improve in anti-HPV56 neutralizing titers, which ranged from 20,480 to 81,920 (5.0 μg group, P < 0.05), 1,280 to 10,240 (1.0 μg group) and 1,280 to 2,560 (0.2 μg group, P < 0.01); these have been all considerably increased than these elicited by the WT HPV66 VLPs (anti-HPV56 titers of 80 to five,120). Taken collectively, most HPV56/66 chimeric VLP not solely enhanced titers towards the heterologous kind but in addition maintained their efficiency in eliciting neutralizing antibodies towards their analogous sorts, demonstrating glorious cross-neutralization capability.
Conversely, chimeric VLPs with HPV53 because the spine exhibited suboptimal particle meeting and diminished cross-neutralization capability (Fig. 4b). However, notable findings emerged: among the many H53-56 VLP variants, solely H53-56HI achieved neutralizing antibody titers towards HPV56 similar to these of WT HPV56, affirming the immunodominant nature of the HI loop for HPV56.Within the case of the H53-66 VLP, the DE loop of HPV66 exhibited superior neutralizing antibody induction on the heterologous framework, mirroring the findings with HPV56 as the inspiration. Nonetheless, chimeras incorporating all 5 HPV66 loops into an HPV53 spine proved to be much less efficient, highlighting the inadequacy of HPV53 as a scaffold for chimeric constructions. Moreover, relating to modifications in immunogenicity particular to HPV53 itself, each H53-56 and H53-66 chimeric VLPs revealed a constant sample whereby substitutions of HPV53’s DE, FG, and HI loops impacted its immunogenicity to various levels, suggesting these loop areas probably maintain extra advantageous positions within the induction of neutralizing antibodies towards HPV53.
We organized the logarithmic fold modifications of the neutralizing antibody titers, which focused each the spine and loop sorts, elicited by high-dose administrations of double-type chimeric VLPs, along side these induced by WT VLP, right into a heatmap (Fig. 4c). This visible illustration successfully underscored that the HI loop of HPV56 constitutes its principal immunogenic loop, modifications within the DE loop of HPV66 are pivotal for adjusting the HPV66 titer, and replacements inside the DE, FG, HI loops of HPV53 considerably affect its immunogenicity. Moreover, we carried out an in depth evaluation of the sequence homology throughout the loop areas of those three HPV sorts, as depicted in Fig. 4d. Remarkably, HPV56 and HPV66 not solely reveal a better stage of full-length sequence homology but in addition exhibit higher amino acid sequence consistency inside their respective loop areas in comparison with these of HPV53, with the consistency of the FG loop between HPV53 and HPV66 similar to that noticed within the HPV56/66 FG loop. Taken collectively, these observations point out that the phylogenetic closeness amongst HPV sorts is inversely associated to the variability in loop areas, thus facilitating the profitable execution of homologous loop substitutions.
Among the many array of twenty double-type chimeric VLPs explored, H56-66DE, H66-56FG, and H66-56HI emerged as frontrunners in cross-type neutralization efficacy, prompting additional analysis of their ED50 values. As illustrated in Desk 1 and Supplementary Desk 2, HPV56 exhibited an ED50 of 0.021 μg towards its personal variant, whereas for HPV66, the ED50 exceeded 0.900 μg. Conversely, HPV66 demonstrated an ED50 of 0.057 μg towards itself and 0.747 μg towards HPV56, aligning with the beforehand famous elevation of HPV66 to HPV56 cross-neutralizing antibody titers. Notably, H66-56HI’s ED50 values signified a outstanding enhancement in cross-immunogenicity versus the person WT VLP, ED50 values inside the discernible vary towards each HPV56 and HPV66, measuring 0.300 μg and 0.100 μg, respectively, highlighting its promise as a possible vaccine candidate for cross-protection research. Thus, H66-56HI is earmarked for prolonged cross-immunization analysis, reflecting its superior immunogenic and cross-neutralization profile.
Triple-type cross-neutralization achieved by incorporating HPV53 and HPV56’s immunodominant loops into HPV66 VLP
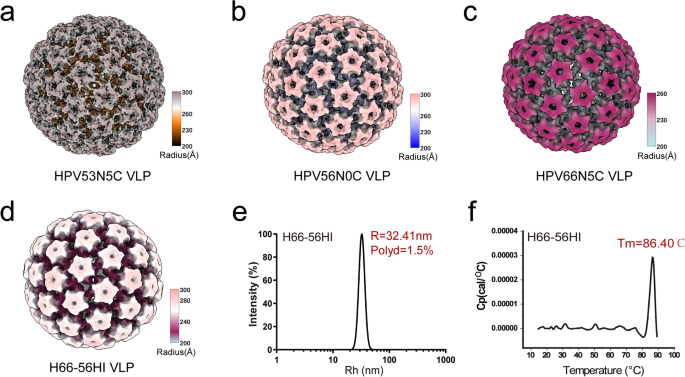
By way of homologous loop swapping between differing kinds, we recognized distinct immunodominant loop areas for HPV53, HPV56, and HPV66, offering a chance for the combination of immunodominant epitopes. We’ve demonstrated that utilizing the HPV66 scaffold can functionally rework the immunodominant loop HI of HPV56, enabling cross-neutralization between the 2 sorts. Cryo-EM confirmed the structural integrity of the H66-56HI capsid and decided the resolutions of HPV53, HPV56, HPV66, and H66-56HI capsids to be 10.12 Å, 10.20 Å, 18.00 Å, and 9.20 Å, respectively. These measurements adopted the established FSC normal of 0.143 (see Fig. 5a–d and Supplementary Fig. 4). The structural properties noticed in H66-56HI have been in keeping with these of the WT VLPs, with DLS quantifying a median particle radius of 32.41 nm (Fig. 5e). Importantly, H66-56HI exhibited higher thermal stability in comparison with WT HPV66, with its Tm peaking at 86 °C (Fig. 5f). This enhanced stability underscores its potential as a strong vaccine candidate.
Cryo-EM structural evaluation and biophysical characterization of WT and H66-56HI VLPs (a–d) Cryo-EM elucidates the intricate structural particulars of WT HPV53 (a), 56 (b), 66 (c), and H66-56HI chimeric VLPs (d), with resolutions of 10.12 Å, 10.20 Å, 18.00 Å, and 9.20 Å, respectively. Radial coloration gradients from 200 Å to 300 Å intensify conformational subtleties. e DLS measurements present particle dimension distribution, and (f) DSC analyzes the thermal stability of the H66-56HI chimeric VLP
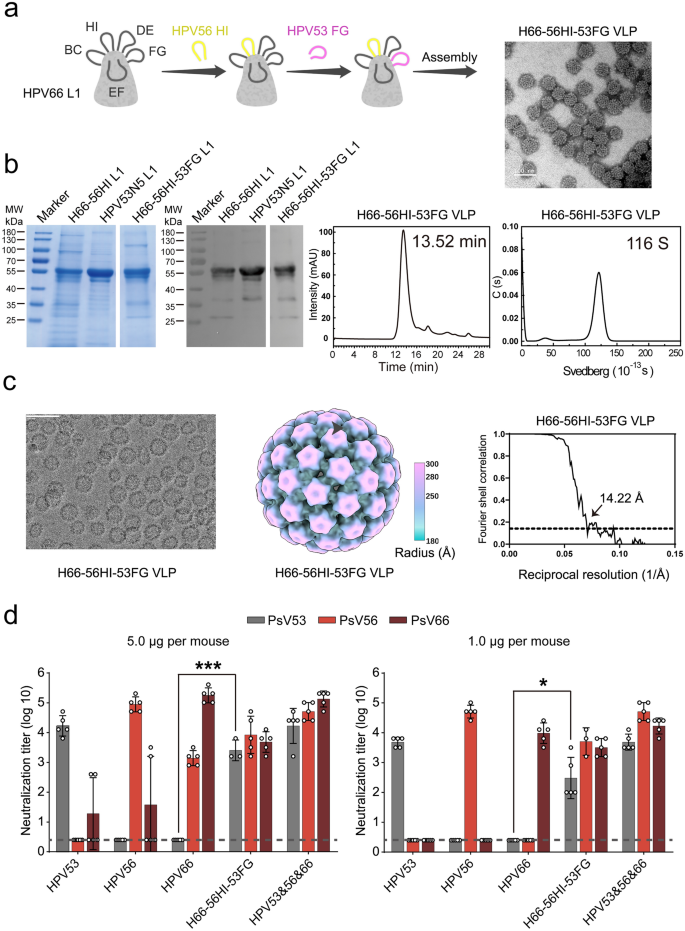
Extending epitope integration throughout a number of HPV sorts resulted within the growth of the tri-type chimeric H66-56HI-53FG VLP. Following normal expression, purification, and in vitro meeting protocols, well-assembled chimeric VLPs have been obtained (Fig. 6a–b). Regardless of present process twin epitope transforming, these VLPs retained structural traits just like the wild-type counterparts. Cryo-EM evaluation revealed that H66-56HI-53FG adopts an everyday icosahedral symmetry (T = 7), similar to HPV53 and HPV66 WT VLPs, with a decision of 14.22 Å (FSC = 0.143), additional demonstrating structural consistency with H66-56HI (Fig. 6c). Immunogenicity analysis in a mouse mannequin (Fig. 6d) demonstrated that H66-56HI-53FG considerably improved neutralizing antibody titers towards exogenous loop sorts (P < 0.05) whereas sustaining its means to induce neutralizing antibodies towards its personal kind. This enchancment was particularly evident within the high-dose group and remained important within the low-dose group. Subsequently, our integration technique combining dominant epitopes with optimized scaffold VLPs efficiently generated cross-immunogens able to neutralizing a number of HPV sorts. This method enhances our understanding of multi-type cross-immunogen design and highlights the potential of complicated antigen design to broaden the protecting scope of HPV vaccines.
Complete evaluation of H66-56HI-53FG VLPs. a Schematic of recombinant chimeric HPV53/56/66 L1 proteins, highlighting the immunodominant loops of WT HPV53 (pink) and HPV56 (yellow) on an HPV66 scaffold (grey), with TEM affirmation (scale bar: 100 nm). b Integrity of the H66-56HI-53FG assemble assessed through lowered SDS-PAGE and western blot utilizing mAb 4B3, with particle uniformity examined by HPSEC elution profiles and AUC sedimentation coefficients. c TEM picture of H66-56HI-53FG VLPs, displaying well-formed particles (scale bar: 100 nm, left panel). Cryo-EM reconstruction of H66-56HI-53FG VLPs reveals an icosahedral construction with radial coloration gradients starting from 180 Å to 300 Å, highlighting key structural options (center panel). The decision of the VLPs was decided to be 14.22 Å, based mostly on the FSC threshold of 0.143 (proper panel). d Immunogenic potential was assessed in BALB/c mice (n = 5) with intraperitoneal injections of VLPs at 5.0 or 1.0 μg/dose at weeks 0, 2, and 4. Neutralizing antibody responses have been measured at week 6 post-initial dose utilizing particular neutralization assays, with sensitivity thresholds indicated by dotted strains. ANOVA evaluation of outcomes, introduced as imply ± SD, reveals statistical significance (*P < 0.05, **P < 0.01; ***P < 0.001; ****P < 0.0001), with error bars and particular person knowledge factors indicating immune response variability