Liposome
The mixture of liposomes and protein supply represents a novel biotechnological strategy with the target of enhancing the soundness and supply effectivity of protein medication. As an artificial membrane-like construction, liposomes exhibit glorious biocompatibility and controllability, enabling shut binding with proteins for focused supply and sustained launch of proteins. The methods wherein liposomes enter cells primarily embody direct fusion with the plasma membrane, entry via endocytosis, and lipid trade with the plasma membrane. These strategies all depend on the interplay and cost matching between liposomes and the cell membrane [26, 27]. The binding modes of liposomes and proteins could be broadly categorised into two classes: encapsulation and modification. Within the context of encapsulation methods, liposomes make the most of their double-layer membrane construction to encapsulate proteins, thereby forming secure liposome-protein complexes. This technique is especially well-suited for the supply of therapeutic proteins, together with glucose oxidase, catalase, horseradish peroxidase, and superoxide dismutase [28,29,30,31]. Proteins equivalent to enzymes are vulnerable to numerous elements within the in vitro surroundings, resulting in structural harm and lack of exercise. As a protecting barrier, liposomes can isolate the exterior surroundings from interfering with proteins, sustaining their stability and exercise. On the similar time, liposomes may present an acceptable microenvironment that helps proteins launch slowly within the physique, extending their motion time [32]. Liposomes can be mixed with polypeptide proteins via modification methods. This technique has huge functions in fields equivalent to vaccine adjuvants [33,34,35]. By way of particular chemical modifications, liposomes can type secure covalent or non-covalent bonds with polypeptide proteins to attain environment friendly loading of proteins. As a provider, liposomes may goal the supply of polypeptide proteins to particular tissues and organs, bettering the immune impact and security of vaccine adjuvants [36].
Liposomes for enzyme supply
Therapeutic enzymes have now turn out to be efficient therapeutic medication for a lot of main ailments and play an necessary function within the remedy of congenital enzyme deficiency. For instance, childish neurofibromatosis, a lysosomal storage dysfunction characterised by the buildup of metabolites in lysosomes as a result of lack of ppt1, can result in the formation of inclusions often called granular halophilic deposits [37, 38]. Enzyme alternative remedy (ERT) helps restore the blocked perform of tissues or cells by supplementing these lacking enzymes, thereby assuaging the illness. Nevertheless, regardless of the potential efficacy of ERT, its translation into medical functions has certainly been hindered by a number of elements. These obstacles might embody enzyme supply, stability, and immunogenicity [39].
Encapsulating enzyme-based proteins inside liposomes addresses particular challenges, together with enhancing their stability and mitigating immunogenicity. Santi et al. described using liposomal supply of ppt1 enzyme for the remedy of childish neurofibromatosis [37]. These liposomes containing enzymes can restore secure ranges of enzyme exercise in fibroblasts of CLN1 sufferers, promote the supply of proteins to the central nervous system, and have an effect on intracellular organic pathways. As well as, the supply of catalytic enzymes, equivalent to glucose oxidase, can assist kill tumor cells (Fig. 2). In earlier analysis, glucose oxidase was encapsulated and delivered into tumor cells by liposomes [32]. The manganese-based nanoprobes NanoMn-GOx-PTX are primarily composed of a manganese core and a phospholipid bilayer shell, which collectively carry glucose oxidase, paclitaxel, and fluorescent dye. The platform is able to releasing manganese ions and payloads in a pH-dependent method inside tumor cells. Subsequently, glucose oxidase catalyzes the manufacturing of hydrogen peroxide from glucose, which is additional catalyzed by manganese ions to provide reactive oxygen species. Mixed with the antitumor impact of paclitaxel, it exhibits sturdy anti-tumor results. As well as, current analysis experiences that Wang et al. developed a liposome-based enzyme nanoreactor, which cleverly encapsulates glucose oxidase GOx and horseradish peroxidase HRP collectively within the aqueous core of liposomes. By way of encapsulation of liposomes, the 2 enzymes can work collectively in the identical house, considerably bettering the effectivity of the complete tandem response. GOx can successfully devour glucose in tumor cells and produce gluconic acid and hydrogen peroxide. The manufacturing of gluconic acid can cut back the pH worth of the native surroundings and improve the focus of hydrogen peroxide, which collectively promote the catalytic effectivity of HRP, ensuing within the manufacturing of extremely cytotoxic hydroxyl radicals ·OH, finally attaining the aim of killing tumor cells [17] .
Liposome-mediated supply of GOx for antitumor remedy. (a) A diagrammatic illustration of the GISLs’ constituent elements and the anticancer mechanism exerted by GOx, dc-IR825, and sorafenib. Reproduced with permission from Ref [40]. (b) A schematic outlining the fabrication of the cell metabolism regulator ATO/GOx PLP and its utilization in most cancers remedy via a PpIX translocation and cell respiration substrate redistribution mechanism. Reproduced with permission from Ref [41]. (c) An outline of a liposomal supply system (SN-38∩LP@Fe3O4/GOx) and a ROS area impact transistor for the enhancement of chemodynamic remedy. Reproduced with permission from Ref [42]
On account of their distinctive catalytic perform, enzymes can’t solely endure colour reactions with their substrates but in addition function instruments for detecting enzyme exercise [43,44,45]. Due to this fact, they’re typically used as mannequin proteins to guage the effectivity of supply instruments. This course of is often achieved by observing fluorescence phenomena or detecting the exercise of enzyme within the cytoplasm. For instance, superoxide dismutase (SOD) has a particular perform of catalyzing the conversion strategy of anionic superoxide radicals in molecular oxygen and hydrogen peroxide. Within the medical area, this enzyme is extensively used, particularly within the remedy of ailments attributable to ROS, equivalent to rheumatoid arthritis, numerous inflammatory ailments, and ischemia-reperfusion damage [46]. Nevertheless, it’s value noting that direct administration of SOD with out the help of an acceptable supply system faces many limitations. Its half-life within the blood is comparatively brief. This makes it tough to successfully accumulate in broken areas and is well filtered by the kidneys [47]. On this regard, the presence of PEG on the floor of liposomes performs a vital function [48]. It may considerably cut back the opsonization of liposomes by the mononuclear phagocytic system, thereby successfully selling the supply impact of SOD-loaded liposomes.
Nonetheless, regardless of the appreciable promise of liposomes in protein supply, the method of encapsulating proteins just isn’t with out shortcomings. A notable drawback is that this course of might probably impair the performance of the proteins in query. Such harm incessantly arises from the intricate procedures concerned in liposome preparation, whereby a number of steps might have a detrimental affect on the construction and performance of proteins [49,50,51]. As an illustration, the pH worth employed throughout liposome preparation represents a pivotal issue. The pH stage exerts a direct affect on the self-assembly strategy of phospholipid molecules, which, in flip, impacts the soundness of the encapsulated proteins. Moreover, the temperature of the answer is a major issue that influences the exercise of liposome-encapsulated proteins. It’s crucial that the temperature of the answer be strictly managed throughout the preparation of liposomes, as this may be certain that the phospholipid molecules can appropriately self-assemble and encapsulate proteins.
Liposomes for Ribonucleoprotein (RNP) supply
Clustered repeatedly interspaced brief palindromic repeats (CRISPR) related protein 9 (Cas9) is a promising gene enhancing instrument for treating ailments on the genetic stage [52]. Nevertheless, the problem of safely and successfully delivering CRISPR/Cas9 to host cells limits its medical software [53]. In comparison with delivering genes, direct supply of Cas9 RNP can instantly perform with out protein expression processes [7, 54,55,56]. The big measurement of Cas9 protein, roughly 160 kDa, prevents it from being immediately delivered into cells. Liposome packaging know-how can’t solely immediately ship Cas9 RNP into cells, but in addition partially shield it from degradation. The power of liposomes to ship intact Cas9 RNP represents a key benefit of this strategy, guaranteeing efficient and non-toxic gene enhancing.
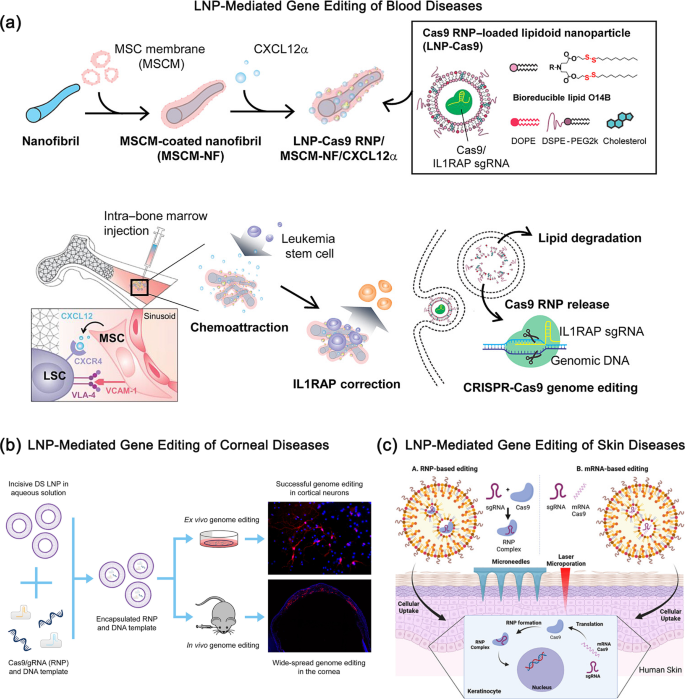
Purposes of Liposome-Facilitated Supply of RNP in Various Illnesses. (a) A lipid nanoparticle (LNP) encapsulated Cas9 RNP supply system is introduced. PCL nanofibrils (NFs), which mimic the bone tissue microenvironment, are coated with mesenchymal stem cell membrane (MSCM) and loaded with the CXCL12α cytokine together with LNP-encapsulated Cas9 RNP. This LNP-Cas9 RNP/MSCM-NF/CXCL12α advanced could be injected into the bone marrow cavity to induce chemotaxis of leukemia blasts or leukemia stem cells (LSCs), enhancing gene enhancing cargo supply efficacy. Reproduced with permission from Ref [57]. (b) CRISPR RNP supply by way of LNPs allows widespread in vivo genome enhancing within the mouse cornea. Reproduced with permission from Ref [54]. (c) A lipid nanoparticle-mediated hit-and-run strategy achieves environment friendly and secure in situ gene enhancing in human pores and skin. Reproduced with permission from Ref [51]
Liposome-delivered gene enhancing protein complexes have been extensively used within the research of varied ailments (Fig. 3), equivalent to gene enhancing in ophthalmology, otology, and dermatology [51, 54,55,56, 58, 59]. Within the area of corneal ailments, Mirjalili Mohanna et al. examined a brand new LNP platform by offering pre-complexed RNPs and template DNA to cultured mouse cortical neurons, and achieved profitable in vitro genome enhancing. Then, the LNP-encapsulated RNP and DNA templates have been immediately injected into the mouse cornea to guage in vivo supply. This research demonstrated intensive genome enhancing within the cornea utilizing LNP-RNPs for the primary time [54]. Within the area of listening to issues, Tao et al. discovered that the in vivo supply of liposome-mediated CRISPR-Cas9 RNP complexes can result in the particular enhancing of Obl alleles [59]. In vivo genome enhancing promotes the survival and restoration of perform in outer hair cells, thereby restoring listening to. By lipid and AAV mediated supply of Streptococcus pyogenes Cas9 (SpCas9) RNP complexes, researchers have improved listening to in mouse fashions of dominant listening to loss with hair cell origin by focusing on the mutant allele of transmembrane channel-like 1 [59]. Within the realm of pores and skin ailments, Bolsoni et al. have investigated the potential of LNP to ship gene enhancing instruments into the dwelling dermis of human pores and skin, enabling environment friendly in situ gene enhancing that might probably remedy uncommon monogenic pores and skin ailments [51]. Due to this fact, the aforementioned research have demonstrated the intensive functions and potential of liposome-delivered gene enhancing protein complexes in numerous illness areas.
To facilitate the medical translation of liposome-based genome enhancing therapies, a number of novel liposome methods with responsive managed launch have been reported for the environment friendly supply of CRISPR-Cas9 RNP into goal cells [56, 60, 61]. Gentle-triggered liposome methods have been used to attain temporal and spatial managed launch of CRISPR-Cas9 RNP [56]. By incorporating photosensitive molecules, equivalent to Verteporfin (VP), into liposomes and exposing them to particular wavelength mild, the liposomes endure structural instability, resulting in the managed launch of RNP for gene enhancing. For instance, Aksoy et al. developed light-triggered liposomes that controllably launch CRISPR-Cas9 ribonucleoprotein by incorporating the clinically used photosensitive molecule VP into the lipid bilayer after which rationally designing it. Beneath 690 nm wavelength mild irradiation, VP reacts with accessible oxygen molecules and generates singlet oxygen, which quickly oxidizes unsaturated lipid elements and results in structural instability of the liposomes and the discharge of ribonucleoprotein [56]. This regulatory mechanism restricts CRISPR-Cas9 activation to designated goal websites, thereby attaining enhanced tissue- and cell-type specificity. Moreover, Yan et al. reported a phosphorylated DNA-engineered liposome system able to responding to stimuli to attain cell-specific intracellular supply and genome enhancing [61]. The liposome design mimics the viral fusion course of, which might set off membrane fusion below pH or UV stimulation to attain cytoplasmic supply of proteins. This technique is very environment friendly in delivering proteins of various sizes and fees to focus on cells. In abstract, these liposome methods supply progressive methods for temporal and spatial management and cell-specific supply of CRISPR-Cas9 RNP, thereby paving the best way for safer and more practical genome enhancing therapies.
Liposomes for fluorescence imaging
Liposome-protein complexes, as environment friendly and exact instruments, are extensively utilized in cell labeling and imaging methods [43, 62]. By combining the wonderful membrane fusion properties of liposomes with the particular recognition capabilities of proteins, scientists are in a position to precisely label and meticulously observe cells on the microscopic stage. This advanced not solely reveals excessive focusing on skill, enabling exact localization to focus on cells or particular intracellular areas, but in addition produces glorious imaging outcomes, clearly revealing the morphology, construction, and useful standing of cells.
The usage of liposome-protein complexes for cell labeling and imaging has enabled scientists to achieve deeper insights into the organic processes occurring inside cells. This encompasses a mess of processes, together with cell development, division, metabolism, and sign transduction. Such understanding not solely elucidates the enigmas of life but in addition furnishes a vital theoretical basis and sensible steerage for the prognosis and remedy of ailments. As an illustration, Li et al. have efficiently developed a multi-faceted ultrasound molecular probe often called cell-penetrating peptide-modified 10-hydroxycamptothecin-loaded phase-transformation lipid nanoparticles, or just iRGD-ICG-10-HCPT-PFP-NPs [63]. When mixed with low-intensity targeted ultrasound, liposome protein probe can be utilized for exact prognosis and remedy of hepatocellular carcinoma. This probe reveals glorious focusing on skill, enabling ultrasound/photoacoustic (PA) dual-mode imaging. It may penetrate deeply into the tumor, attaining a greater therapeutic impact, and thus supplies new concepts and strategies for the prognosis and remedy of liver most cancers. Three-dimensional optical microscopy performs a vital function in understanding and optimizing the supply of nanomedicines [64]. Nevertheless, sadly, the method of tissue clearing typically removes liposomes, stopping the method from attaining three-dimensional imaging of liposomes inside tissues. Fortuitously, Professor Warren C. W. Chan designed a protein tag named REMNANT, which can’t solely connect to liposomes but in addition crosslink with tissues whereas remaining secure throughout the clearing course of, thus enabling three-dimensional imaging of liposomes in intact tissues [25]. The REMNANT tag may monitor the discharge fee of liposome contents in tissues in actual time. This progressive technique not solely helps researchers observe the habits of degradable supplies in vivo, but in addition supplies beneficial steerage for the engineering design of imaging methods and drug supply autos.
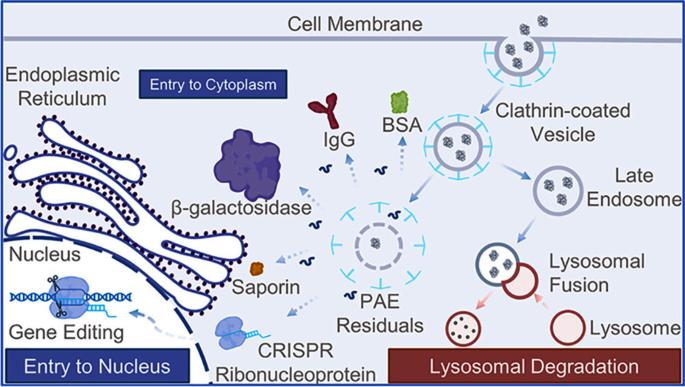
Redirecting host cell signaling pathways via the utilization of bacterial effectors by way of cationic lipid-mediated intracellular protein supply. Reproduced with permission from Ref [65]
Moreover, fluorescent proteins have been efficiently utilized by researchers together with liposome supply know-how to focus on proteins, enabling clear statement of the absorption effectivity and subcellular localization of nanoparticles, offering new views and instruments for analysis in nanomedicine and drug supply [65,66,67]. Yang et al. have reported using a high-throughput liposome screening technique to efficiently obtain intracellular supply of OspF mediated by cationic liposomes [65]. This technique successfully particularly inhibits the MAPK signaling pathway and tumor development in most cancers cells, in addition to particularly regulates the immune response of macrophages. To additional enhance the encapsulation effectivity of lipid nanoparticles throughout intracellular supply, researchers genetically fused OspF with a negatively charged inexperienced fluorescent protein. This fusion promotes the self-assembly of cationic lipid nanoparticles via electrostatic interactions, and supplies sturdy help for learning protein transport habits on the mobile stage. Total, the applying of liposome-protein complexes has not solely deepened our understanding of liposome supply mechanisms, but in addition opened up new prospects and potential functions for future drug supply and illness remedy fields (Fig. 4).
Protein nanoparticles
Lately, the cytosolic supply of protein medication via protein nanoparticles has emerged as a major breakthrough within the area of biomedicine [68, 69]. This know-how makes use of single or a number of protein molecules to type nanoparticles with nanoscale dimensions via self-assembly, enabling environment friendly cytosolic supply. This part will give attention to three progressive protein nanoparticle design strategies. These strategies not solely exhibit the potential of protein nanoparticles within the biomedical area, but in addition present new views and techniques for the event of cytosolic supply know-how. The next is a quick overview of those three design strategies: The primary is the design of protein nanoparticles primarily based on phase-separated condensates. This technique makes use of the interactions between completely different proteins and controls environmental situations equivalent to temperature, pH, or ionic energy to induce section separation and subsequent formation of condensates [70, 71]. These condensates function drug carriers or imaging instruments to carry out particular capabilities inside cells. A second progressive design strategy is using computer-designed protein nanoparticles. The development of bioinformatics and computational biology has enabled the utilisation of laptop simulations and algorithm optimisation within the design of protein nanoparticles with outlined buildings and capabilities. This technique permits for exact management over the scale, configuration, and floor traits of nanoparticles, thereby enabling exact regulation of the drug supply course of. Furthermore, computer-aided design can predict the interactions between nanoparticles and cells, thereby offering beneficial perception for optimizing supply effectivity and minimizing uncomfortable side effects. The third class of protein nanoparticles relies on cell-penetrating peptide nanocarriers. Cell-penetrating peptides are brief peptides that possess the capability to traverse mobile membranes. The mixture of cell-penetrating peptides with proteins permits for the creation of peptide-modified nanocarriers, which might facilitate the transmembrane supply of proteins in an environment friendly method. These progressive design strategies for protein nanoparticles possess distinctive traits, offering new views and superior instruments for developments in cytosolic protein supply know-how.
Section-separated protein self-assembly condensates
Section-separated protein self-assembly condensates supply is a cutting-edge biotechnology that makes use of the section separation properties of proteins below particular situations to type condensates via self-assembly, thereby attaining focused supply of proteins. Section separation is a course of the place intracellular proteins or protein-RNA complexes spontaneously type distinct “phases,” involving each enticing and repulsive interactions amongst proteins, in addition to thermodynamic driving forces [72, 73]. Condensates type inside cells sometimes via spontaneous meeting by way of interactions amongst biomacromolecules equivalent to proteins and RNAs. These condensates are usually not meant to limit molecular diffusion however slightly to supply a regionally high-concentration surroundings to facilitate particular intermolecular interactions. Throughout the condensates, interactions amongst molecules, equivalent to electrostatic and hydrophobic interactions, trigger them to tightly cluster collectively. Nevertheless, this clustering just isn’t utterly enclosed however slightly possesses a sure diploma of permeability. Due to this fact, molecules can nonetheless diffuse to a sure extent throughout the condensates and between the condensates and the encompassing cytoplasm [74].
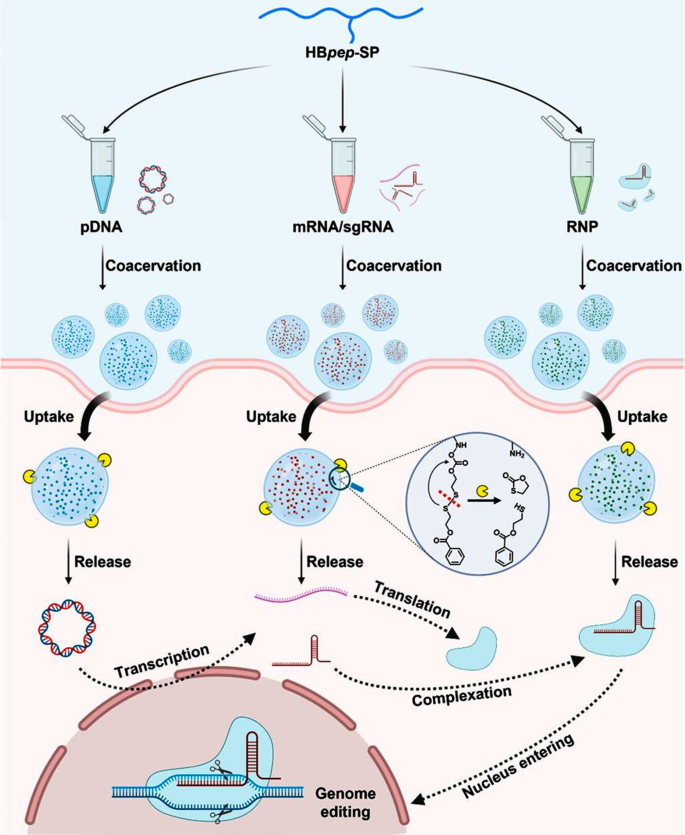
The condensates encapsulate proteins inside their inside and ship them to particular goal areas via intracellular transport mechanisms [75]. The method of intracellular transport of phase-separated condensates might contain the next mechanisms: direct membrane translocation and entry into the cell via classical endocytosis. Direct membrane translocation refers back to the mode of protein supply throughout the cell that avoids seize by endosomes/lysosomes, immediately fusing with the membrane to move the cargo into the cytoplasm. For instance, redox-responsive peptide(HBpep) coacervates and magnetically responsive peptide (DgHBP-2) coacervates [76, 77]. Section-separated condensates can be internalized by cells via energy-dependent endocytosis [78, 79].The classical endocytosis pathway primarily consists of the macropinocytosis pathway, the clathrin-mediated endocytosis pathway, and the caveolin-mediated endocytosis pathway [80]. Some condensates are internalized via endocytosis to ship coupled anticancer medication to focus on cells. For instance, Dittrich et al. developed Flutax-2 peptide nanoparticles functionalized with transferrin, which enter cells via clathrin-mediated TfR endocytosis [79]. After coming into the cytoplasm, condensates could be transported by molecular motors hooked up to microtubules or microfilaments [81]. Moreover, condensates within the cytoplasm can also be encapsulated inside vesicles, enabling their transport inside and out of doors the cell via the fusion and separation processes of the vesicles with the cell membrane. For instance, phase-separated YBX1 condensates recruit miRNAs and selectively type them into exosomes [82]. In comparison with different supply autos, equivalent to liposomes and polymeric nanoparticles, protein condensates type nearly instantaneously, exhibiting negligible cytotoxicity from their peptide constructing blocks and eliminating the necessity for natural solvents which will cut back the organic exercise of encapsulated molecules. This ensures each effectivity and security within the supply course of.
Section-separated protein peptides are able to loading macromolecules, traversing cell membranes, and delivering their payloads inside cells, thereby overcoming the foremost limitation of the problem in intracellular supply of macromolecules (Fig. 5). Yu et al. reported a research on glucose-driven droplet formation in supramolecular peptide and therapeutic protein complexes. The research revealed that below particular situations, these complexes can reply to glucose stimulation, resulting in section separation and droplet formation. This discovery not solely supplies a brand new perspective for understanding intermolecular interactions amongst biomolecules but in addition opens up new avenues for drug supply and organic remedy [20]. In one other research, Solar et al. developed brief His-rich, pH-responsive beak peptide (HBpep) coacervates that bind to disulfide bonds containing self-sacrificial fragments (HBpep-SR), which might set off the disintegration of droplets in a lowering surroundings, selling the intracellular supply of protein medication [76]. HBpep condensates can cross cell membranes independently of endocytosis, demonstrating their potential for intracellular supply of therapeutic brokers. At low pH, histidine-rich HBpep exists as monomers, however quickly section separates into condensed microdroplets at impartial pH, throughout which course of it could possibly take in and combine numerous macromolecules from the answer. These examples absolutely exhibit the flexibility and practicality of phase-separated protein self-assembly condensates within the supply of therapeutic proteins.
At the moment, the know-how of phase-separated protein self-assembly condensates for protein supply has demonstrated potential software worth in a number of fields, equivalent to drug supply, gene remedy, and cell regeneration [83, 84]. Nevertheless, regardless of these developments, there are some points dealing with phase-separated protein self-assembly condensates, primarily centering on the soundness, focusing on, and systematic analysis of the condensates. In comparison with stable particles, the droplets fashioned by protein condensates pose sure challenges by way of stability. Particularly, these droplets could also be extra vulnerable to environmental elements throughout the supply course of, resulting in their decomposition earlier than reaching the goal cells or tissues. This instability might cut back the therapeutic effectivity and even have an effect on the general end result of the remedy. Future analysis must give attention to exactly controlling the discharge kinetics of the embedded proteins and designing protein droplets with larger cell and tumor specificity [85]. Researchers may make the most of biomarkers and different means to additional optimize the focusing on of protein condensates, attaining extra exact therapeutic results. Notably, there may be nonetheless inadequate systematic analysis on protein condensates in vivo, and it’s essential to give attention to evaluating their security, effectiveness, and tissue distribution within the physique [86]. With additional in-depth analysis and steady optimization of this know-how, it’s believed that it’ll play a extra vital function within the area of biomedicine sooner or later.
Redox-Responsive Section-Separating Peptide as a Common Supply Car for CRISPR/Cas9 Genome Enhancing Equipment. Reproduced with permission from Ref [87].
Self-assembling protein nanoparticles
Within the early 2000s, researchers started using computational strategies to design proteins with particular capabilities. Scientists employed refined algorithms and simulation software program to foretell and optimize protein sequences with a view to obtain the specified nanostructures. These simulations thought-about the interactions between protein molecules, folding, and meeting mechanisms to ensure that the ultimate designed nanoparticles exhibited optimum stability and performance [88, 89]. Upon completion of the design, scientists might make use of genetic engineering methods to synthesize the proteins and induce self-assembly into nanoparticles below appropriate situations. These nanoparticles can exhibit a wide range of morphologies, together with spherical, rod-like, and tubular, and could be personalized based on particular necessities [90,91,92,93]. The possible functions of computer-designed self-assembling protein nanoparticles are intensive, spanning fields equivalent to biomedicine, drug supply, and nanomaterials [94, 95]. For instance, they are often utilized as drug carriers to facilitate the exact supply of medication to focus on tissues or cells, thereby enhancing therapeutic efficacy whereas lowering the incidence of hostile results [96]. Moreover, these nanoparticles can be employed within the building of biosensors, tissue engineering scaffolds, and novel nanoelectronic units [97]. These research present a vital basis for the event of novel biomedical functions (Fig. 6).
David Baker is an esteemed scientist and chief within the area of protein design, having made vital contributions to quite a few areas of analysis, together with protein folding prediction, protein-small molecule binding, self-assembling protein nanoparticles, and protein design [90,91,92, 94, 95, 98,99,100]. Just lately, David Baker’s workforce has developed a reinforcement learning-based strategy to protein construction design, which is named the “top-down design technique“ [100]. This technique is able to designing advanced protein nanomaterials with particular system properties, together with: [1] Disk-shaped nanopores: Utilizing the Monte Carlo Tree Search (MCTS) technique, the researchers crammed the house between two beforehand designed ring-shaped proteins, producing a disk-shaped construction with a central nanopore [2]. Extremely-compact icosahedra: Using the MCTS technique, the researchers created icosahedra with ultra-compact buildings that don’t exist in nature and are smaller and have decrease porosity than identified protein icosahedra. These icosahedra have the potential to show immunogenic and signaling molecules at densities which might be considerably greater than these noticed in naturally occurring buildings. This might result in enhanced vaccine responses and the induction of angiogenesis [3]. Icosahedra of fusion proteins: The researchers fused useful protein domains, such because the angiopoietin 1 F-domain, to the termini of icosahedra, producing bioactive nanoparticles. These particles have demonstrated potent results in activating cell signaling pathways and vaccine functions. The highest-down design technique can handle design challenges which might be intractable with conventional strategies and generate distinctive buildings that don’t exist in nature, enabling the design of advanced protein nanomaterials with particular system properties. In conclusion, this research introduces novel ideas and methodologies to the sphere of protein design, whereas concurrently paving the best way for brand spanking new avenues of inquiry in biomedical analysis and functions.
Pc-designed self-assembly protein nanoparticles. (a–c) In vitro meeting evaluation signifies cooperativity in most regimes. Reproduced with permission from Ref [101]. (d) Pc-Aided Design of Lasso-like Self-Assembling Anticancer Peptides with A number of Features for Focused Self-Supply and Most cancers Remedies. Reproduced with permission from Ref [34]
Previous analysis strategies embody physical-based design strategies like Rosetta, which require a considerable amount of computing sources and handbook refinement by skilled structural biologists. To simplify protein-protein interface design and make it relevant to a variety of scientific issues, de Haas et al. used the deep studying technique ProteinMPNN to design two-component tetrahedral protein nanomaterials [99]. These nanomaterials encompass two distinct trimer constructing blocks which might be organized on the three axes of tetrahedral symmetry, forming a construction often called “T33”. The research designed protein nanomaterials able to environment friendly self-assembly, which might assemble from independently purified elements in vitro, essential for biotechnological manufacturing. Through the use of the deep studying technique ProteinMPNN to design protein-protein interfaces, the researchers overcame the restrictions of conventional physics-based Rosetta design strategies by way of computational effectivity and handbook adjustment. Importantly, the interfaces designed by ProteinMPNN exhibit enhanced polarity, which facilitates seamless meeting of nanomaterials in vitro, essential for environment friendly biotechnological manufacturing. Due to this fact, deep studying can allow extra individuals to take part in protein interface design. The superior AI know-how will promote the event of the subsequent technology of protein-based applied sciences sooner.
Developing protein nanomaterials typically entails the docking and fusion of protein monomers or cyclic oligomers. Nevertheless, as a result of irregularity of protein buildings, these strategies typically face quite a few challenges and difficulties when making an attempt large-scale meeting primarily based on easy geometric ideas. Huddy et al. developed a easy and scalable protein nanomaterial design technique to deal with the restrictions of present strategies by way of geometric regularity in protein meeting. This technique achieves the regularity and scalability of protein nanomaterials via easy geometric guidelines [98]. They launched a novel protein design technique that simplifies the design of multi-component protein assemblies by utilizing standardized protein constructing blocks and demonstrates how these constructing blocks can be utilized to design assemblies starting from easy polygonal and round oligomers to giant polyhedral nanocages and unbounded linear “prepare observe” assemblies, with adjustable sizes and geometric shapes that may be simply blueprinted. The strategy of simplifying protein meeting design via the design of scalable protein constructing blocks supplies new concepts for the design and building of protein nanomaterials.
Pc-designed self-assembly protein nanoparticles characterize a pioneering nanotechnology that integrates the tenets of laptop simulation and protein engineering. This strategy goals to manufacture nanostructures with outlined shapes, dimensions, and functionalities. This know-how allows scientists to attain self-assembly processes on the nanoscale by exactly controlling the sequence and construction of proteins, thereby getting ready nanomaterials with distinctive properties. Nonetheless, regardless of the appreciable promise of computer-designed self-assembly protein nanoparticles, a number of challenges stay to be addressed, together with enhancing manufacturing effectivity, optimizing stability, and guaranteeing biocompatibility. It’s anticipated that, with the continuing development of know-how and additional analysis, these challenges shall be progressively addressed, thereby facilitating the exploration of recent frontiers within the fields of nanomedicine and nanotechnology.
Cell penetrating peptide-modified nanocarriers
Cell-penetrating peptides (CPPs) are a category of brief peptides able to carrying macromolecules into cells. These peptides have obtained widespread consideration as a consequence of their distinctive skill to penetrate cell membranes, significantly within the fields of drug supply and biomedical functions [102]. Over the previous 5 years, quite a few CPPs have been developed and validated for his or her skill to successfully ship bioactive molecules each in vitro and in vivo. As of now, the CPPsite 2.0 database has publicly listed 1,855 distinct CPPs, which could be subdivided into three main classes primarily based on their properties: amphipathic peptides, cationic peptides, and hydrophobic peptides [103]. CPP is usually utilized as a way to switch nanocarriers, with the goal of enhancing their mobile uptake effectivity and transport capabilities [104, 105]. CPP can sometimes be hooked up to the floor of nanoparticles by way of electrostatic interactions or via using covalent coupling methods (Fig. 7).
This schematic illustrates the method of modifying the floor of nanoparticles utilizing cell-penetrating peptides (CPP) via electrostatic or covalent coupling methods, and descriptions its primary benefits and drawbacks. Reproduced with permission from Ref [106]
The supply of CPP-modified nanocarriers throughout cells could be divided into two modes: direct translocation and endocytosis: [1] CPP-mediated membrane translocation: Some CPPs could be embedded in lipophilic cell membrane buildings as a consequence of their distinctive amphipathic properties, which allows them to immediately cross membrane limitations [107]; [2] CPP-mediated membrane pore formation: CPP briefly disturbs the lipid bilayer of the cell membrane, inflicting minor harm to the native membrane construction. This transient membrane pore supplies a passive diffusion pathway for CPP and its cargo molecules to cross the broken cell membrane barrier [108]; [3] CPP-mediated endocytosis: CPP or its cargo-carrying complexes have additionally been proven to enter the inside of cells via an energy-dependent course of referred to as endocytosis [109]. This course of encompasses a number of endocytic pathways, equivalent to pinocytosis, caveolin-mediated endocytosis, and clathrin-mediated endocytosis, that are primarily chargeable for mobile uptake of comparatively giant protein complexes [110].
In mucosal tissue areas such because the oral cavity and lungs, CPP-modified nanocarriers can considerably promote the interplay between the drug provider and the mucus layer or epithelial tissue, thereby successfully prolonging their residence time on the goal web site [102, 109]. For instance, Rehmani et al. launched a novel nanocarrier modified with CPP, often called Glycosaminoglycan-binding-enhanced-transduction (GET), which capabilities as an environment friendly transepithelial supply vector in vitro and facilitates oral insulin exercise in diabetic animals [111]. By using electrostatic interactions, insulin could be linked to GET to create nanocomplexes (Insulin GET-NCs). These nanocomplexes considerably increase insulin transport in vitro inside differentiated intestinal epithelium fashions, demonstrating a greater than 22-fold improve in translocation.
CPP-modified nanocarriers have additionally demonstrated exceptional potential in crossing the blood-brain barrier. Many anticancer medication are hindered by the blood-brain barrier and discover it tough to penetrate into the mind [112, 113]. Nevertheless, with its distinctive cell-penetrating skill, CPP-modified nanocarriers have opened up a brand new pathway for the remedy of mind ailments. For instance, Barra et al. used liposomes functionalized with viral fusion peptide to guage the passage of a neuroprotective agent (pituitary adenylate cyclase-activating polypeptide) via a dynamic in vitro mannequin of the blood–mind barrier [114]. Though CPP can be utilized to successfully ship proteins into host cells, its instability in serum typically confines it to degradation inside endosomes and lysosomes. As soon as transduced into cells, it could possibly induce excessive ranges of cytotoxicity, resulting in suboptimal organic outcomes. To beat this difficulty, the introduction of polypeptide sequences for lysosomal escape can enhance the effectivity of cytosolic protein supply [115]. Moreover, it’s mandatory to pick out applicable CPP sequences, lengths, and modification strategies, in addition to to make adaptive changes primarily based on particular cell varieties and exterior environmental situations.
Polymers
Useful polymer-mediated biomacromolecular supply occupies an necessary place within the area of protein supply and has lengthy been a spotlight of consideration. Pure polymers equivalent to chitosan [116], alginic acid [117], cyclodextrin [118], and chondroitin sulfate [119], in addition to polymeric polymer supplies equivalent to PLGA [120]and PEI [121], can be utilized for protein supply as a consequence of their good biocompatibility and biodegradability. A substantial variety of glorious articles have been revealed introducing these supplies, and they won’t be elaborated on this overview [122,123,124]. Polymer-protein complexes ready via intermolecular interactions typically exhibit instability and are liable to decomposition in buffered options, which is a crucial problem that must be overcome on this area. In contrast with nucleic acids equivalent to DNA and RNA, proteins have a comparatively low internet cost, which undoubtedly will increase the problem of forming secure complexes with polymer carriers. To efficiently put together protein complexes with good stability, we urgently must discover the efficient software of cationic polymers. Grafting completely different useful ligands onto cationic polymers can considerably improve the binding affinity between polymers and proteins and successfully cut back cost repulsion between cationic polymers throughout the formation of polymer/protein complexes. On this part, we are going to delve into the newest progress of polymers modified with completely different useful ligands in protein cytosolic supply, aiming to supply helpful references and inspirations for the longer term growth of this area.
Fluorinated polymers
Fluorinated polymers, with their distinctive ionic interplay capabilities, stand out amongst numerous supplies and are extensively regarded by researchers as a perfect selection for binding to the anionic areas of proteins [125, 126]. These polymers can assemble synergistically via fluorophilic results to type secure nanoformulations, a property that has extraordinarily broad functions within the biomedical area. Such nanoformulations can’t solely shield proteins from exterior environmental influences but in addition successfully ship them to focus on areas, offering new potentialities for the remedy of ailments. The intelligent introduction of fluorine chains into polymers confers twin hydrophobic and lipophobic properties to the polymers, enabling them to encapsulate proteins extra exactly whereas sustaining their exercise. In line with related experiences, the Cheng Yiyun analysis group was the primary to disclose the “fluoroamphiphiles” phenomenon of fluorinated polymers in protein cytosolic supply [125]. They efficiently grafted completely different fluorinated small-molecule compounds onto polyethylenimine, establishing a wealthy library of fluorinated polymer supplies. This discovery opened a brand new path for the applying of fluorinated polymers within the biomedical area. To validate the effectiveness of fluoroamphiphiles, the analysis workforce additional carried out intensive testing utilizing mannequin proteins with various molecular weights and cost properties. The experimental outcomes demonstrated that these fluorinated polymers can effectively ship unmodified proteins into cells whereas avoiding poisonous harm to the cells. This attribute makes fluorinated polymers extremely promising for functions within the biopharmaceutical area.
Notably, the protein supply effectivity of fluorinated polymers is intently associated to the size and fluorination diploma of the PFL chain. Because the fluorination diploma will increase, the supply effectivity additionally improves; nevertheless, for fluorinated polymers with longer PFLs, excessively excessive fluorination levels can truly result in a lower in protein supply effectivity. This discovering supplies necessary steerage for optimizing the design of fluorinated polymers. The hydrophobic properties of fluoroalkyl chains on polymers endow them with excessive membrane affiliation affinity, which permits fluorinated polymers to bind extra simply with cell membranes, thus attaining environment friendly protein supply [127, 128]. The lipophobic property successfully prevents the amphiphilic polymers from fusing with phospholipids throughout endocytosis or direct membrane translocation, guaranteeing the precision and effectivity of the supply course of. In comparison with non-fluorinated analogues mixed with aliphatic lipids, fluoropolymers exhibit vital efficiency benefits, together with excessive tissue permeability and efficient cytoplasmic supply capabilities [129, 130]. They will quickly penetrate deep into tissues and precisely ship medication to focus on areas, thereby enhancing remedy effectiveness [131, 132] (Fig. 8). Nevertheless, positively charged fluorinated polymer/protein complexes are sometimes quickly cleared by the reticuloendothelial system throughout systemic supply. To handle this difficulty, researchers cleverly make the most of anionic polymers to protect the constructive fees on the floor of the complexes [126]. This progressive software technique not solely enhances the soundness of the complexes within the bloodstream but in addition improves their biocompatibility, opening new avenues for the additional growth of fluorinated polymers within the area of biomedicine.
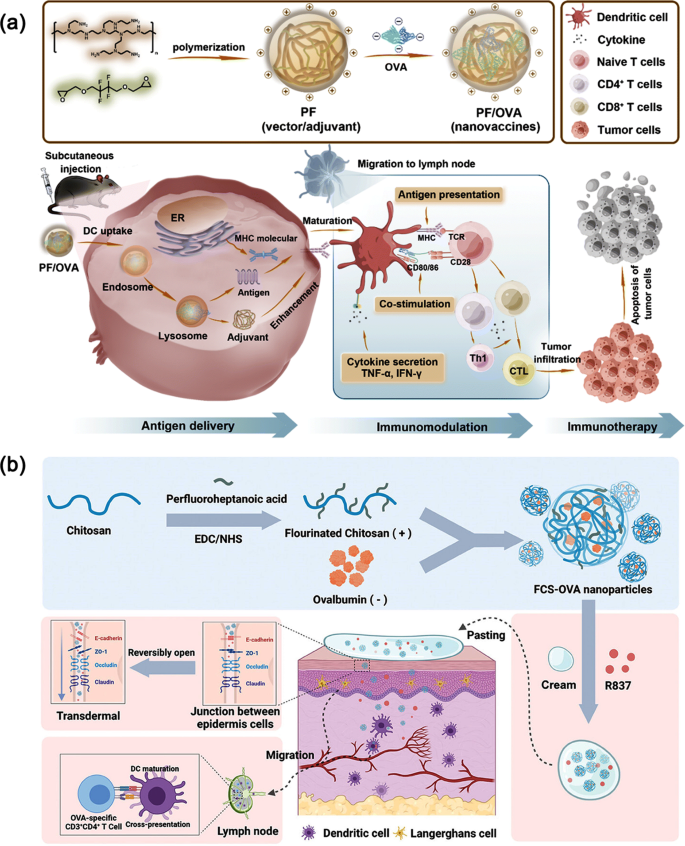
Utilizing Fluorinated Polymers as Supply Vectors for Most cancers Immunotherapy. (a) Illustrative diagram of the manufacturing of fluoropolymer PF, creation of PF/OVA nanovaccines, and the PF/OVA-facilitated immunotherapy process. Reproduced with permission from Ref [133]. ; (b) A non-invasive and needle-free most cancers vaccine software in cream patch type, using fluorinated chitosan as the bottom materials. Reproduced with permission from Ref [134].
Boronated polymers
Boronated polymers have efficiently garnered widespread consideration within the biomedical area as a consequence of their distinctive N-B coordination mechanism [135]. The applying of boronated polymers in drug supply reveals glorious efficiency and potential. Firstly, boronated polymers can exactly determine tumor cells as a consequence of their excessive affinity for sialic acid residues on cell membranes [136]. This recognition skill permits boronated polymers to selectively bind to tumor cells, thus avoiding harm to regular cells [137]. Secondly, via a number of mechanisms equivalent to nitrogen-boronic acid coordination, guanidinium-π interplay, and ionic interplay, boronated polymers obtain deep binding with cationic and anionic areas on the protein floor [138]. This deep binding not solely enhances the interplay between medication and cells but in addition improves the soundness and bioavailability of medication [139]. As well as, boronated polymers additionally exhibit low cytotoxicity and glorious serum stability [135]. Because of this throughout drug supply, boronated polymers won’t trigger harm to regular cells whereas sustaining stability in advanced organic environments. Due to this fact, boronation has turn out to be a common and dependable technique for polymer functionalization, offering sturdy help for drug supply and tumor remedy (Fig. 9).
Reversible Nanocomplex Formation for Intracellular Protein Supply with Enhanced Serum Stability. (a) Using a Heterobifunctional Adaptor B1 to Assemble Cargo Proteins and Polycatechols (PC) into Serum-Steady Nanoparticles. (b) Treating Retinal Ischemia/Reperfusion (I/R) Harm via Intravitreal Injection of SOD Nanoparticles Ready by way of the Reversible Meeting Supply System. Reproduced with permission from Ref [135].
For instance, phenylboronic acid (PBA), as a typical electron-deficient group of boronated polymers, performs a vital function within the software of those polymers. PBA can type secure nitrogen-boronic acid coordination with main amines and imidazoles wealthy in lone pair electrons, thereby attaining environment friendly binding with proteins [140]. Moreover, the guanidinium a part of arginine on proteins may tightly bind to PBA via guanidinium-π interplay, additional enhancing the affinity between boronated polymers and proteins. When PBA is grafted onto cationic polymers with a excessive density, these polymers can obtain deep binding with cationic and anionic areas on the protein floor via a number of interplay mechanisms. This deep binding not solely improves the interplay between medication and cells but in addition enhances the focusing on skill and therapeutic impact of medication. Nevertheless, for polymers with comparatively low PBA grafting charges, bettering their affinity with proteins turns into a problem. To handle this difficulty, researchers cleverly launched pure polyphenols. Pure polyphenols are wealthy in phenolic hydroxyl teams and fragrant rings, enabling them to type sturdy hydrogen bonding and hydrophobic interactions with organic molecules equivalent to proteins and nucleic acids. This interplay can compensate for the restrictions of inadequate PBA grafting charges, thus bettering the effectivity of boronic acid-modified polymers. Boronate-modified polymers can obtain particular binding with catechin teams on the protein floor via catechin-boronic ester bonds. This dynamic covalent bond stays secure below physiological situations however could be cleaved in acidic microenvironments. This characteristic permits for exact management of the boronate-modified polymers throughout drug supply, guaranteeing drug launch on the goal location, thereby enhancing therapeutic results and lowering uncomfortable side effects. Research have proven that the addition of pure polyphenols will increase the effectivity of boronate-modified dendrimer polymers by not less than 5 instances [135]. This achievement opens new avenues for drug supply and tumor remedy, probably offering a extra environment friendly and secure drug supply system for future medical therapies.
Guanidinium-functionalized polymers
Guanidinium-functionalized polymers have demonstrated exceptional potential in biomedical functions. On account of their distinctive chemical properties, these polymers considerably improve their binding skill with proteins and successfully promote interactions between cell membranes, resulting in elevated intracellular uptake [141]. This attribute makes guanidinium-functionalized polymers promising candidates for protein supply, drug supply, and cell imaging. Firstly, guanidinium, as a strongly primary group with a pKa worth of 13.6, reveals superior alkalinity amongst completely different amino acids. In comparison with arginine (with an R-group pKa of 12.5) and lysine (with an R-group pKa of 10.5), guanidinium shows a extra distinguished basicity. Consequently, guanidinium can type strong salt bridges and hydrogen bonding interactions with carboxyl teams of glutamic acid or aspartic acid in proteins [142]. This interplay not solely enhances the soundness of polymer-protein binding but in addition facilitates the intracellular localization and distribution of the polymers.
Guanidinium-rich peptides, polymers, and nanoparticles have garnered vital consideration as a consequence of their exceptional membrane translocation exercise. These supplies can quickly bind to cell membranes and enter cells via endocytosis, enabling environment friendly protein or drug supply. Wang et al. modified poly (β-amino ester) (PAE) with phenylguanidine teams to boost its applicability in cytosolic protein supply (Fig. 10). The guanidine-rich PAE exhibited sturdy protein binding skill and excessive protein internalization effectivity, efficiently delivering CRISPR-Cas9 RNP to HeLa cells expressing GFP and attaining over 80% GFP expression knockout [143]. The phenylbiguanide conjugated polymers have exhibited greater exercise in protein binding and polymer internalization. In comparison with monoguanidine polymers, the phenylbiguanide conjugated polymers have demonstrated greater effectivity in protein supply. This polymer not solely has a stronger skill to bind to proteins but in addition can extra successfully promote cell membrane interactions and inner uptake. This discovery supplies a brand new technique for growing extra environment friendly and safer protein supply methods. Notably, regardless of the upper cost density of biguanidines in comparison with monoguanidines, surprisingly, the ensuing polymers exhibit decrease toxicity. The precise causes for this discovering nonetheless want additional investigation, however it might present necessary clues for the event of novel low-toxicity and high-efficiency protein supply vectors.
Schematic illustration of Guanidyl-Wealthy Poly (β Amino Ester) for Common Useful Cytosolic Protein Supply and Gene Enhancing. Reproduced with permission from Ref [143]
Heterocyclic polymers
This regulatory functionality is especially essential for the supply of cytosolic proteins, because it ensures their stability and exercise throughout the supply course of. Heterocyclic natural compounds occupy a pivotal place within the growth of organic macromolecule supply methods. The heteroatoms equivalent to N, O, and S in these compounds, with their notable excessive electronegativity, work together with the heterocyclic rings via inductive results, thereby fine-tuning the cost distribution of the heterocycles. This distinctive property endows heterocyclic polymers with inherent benefits in optimizing the supply effectivity of organic macromolecules. As an illustration, functionalized polyethylenimines and polyethylene glycols can exactly regulate cost and cargo binding capabilities via the modulation of coordination and hydrogen bonding [16, 18, 59, 144,145,146].
Polymer-Coated Upconversion Nanoparticles (UCNPs) with Ligand Alternate and Functionalization for Protein Supply. (a) Fabrication of functionalized polymer-coated upconversion nanoparticles (UCNPs) by way of ligand trade with oleic acid coating. (b) TEM Characterization of B75 polymer-coated UCNPs with detrimental staining. (c) Photoluminescence imaging of UCNPs below 980 nm Excitation (800 mW Laser). (d) TEM imaging of the UCNP-polymer composite methods. Reproduced with permission from Ref [147]
Within the biomedical area, polyethylene glycol (PEG) has intensive functions in organic conjugation, drug supply, floor functionalization, and tissue engineering [20, 32, 48, 148]. For instance, PEGylation covalently {couples} PEG with proteins, considerably bettering the pharmacokinetic properties of peptides and proteins. This enchancment consists of enhanced solubility, extended stability, and diminished immunogenicity, significantly enhancing their software effectiveness inside organic methods [149]. Notably, pyridine thiourea-modified polyethylenimines exhibit distinctive efficiency in cytosolic protein supply [145]. They will tightly bind to cargo proteins like GFP via a mixture of ionic and hydrophobic interactions, guaranteeing that the proteins don’t detach or lose exercise throughout supply. Furthermore, heterocyclic polymers could be mixed with different biocompatible supplies equivalent to liposomes and nanoparticles to type supply methods with particular capabilities. These methods not solely allow focused supply of proteins but in addition improve their stability and bioavailability in vivo. It’s value noting that regardless of the large potential of heterocyclic polymers in organic macromolecule supply, they nonetheless face some challenges in sensible functions. As an illustration, exact management over the synthesis and modification processes of heterocyclic polymers is important to make sure good biocompatibility and stability [129, 149]. For instance, Malhotra et al. achieved long-term stability of nanoparticles utilizing a biocompatible phosphonate-based polymer coating [147]. Moreover, optimizing the design and preparation processes of supply methods is essential to boost their supply effectivity and specificity. Heterocyclic polymers play a major function within the growth of organic macromolecule supply methods (Fig. 11). By way of additional investigation of their properties and software mechanisms, mixed with superior preparation methods and analysis strategies, it’s anticipated that extra environment friendly and secure organic macromolecule supply methods shall be developed sooner or later, making vital contributions to the development of the biomedical area.