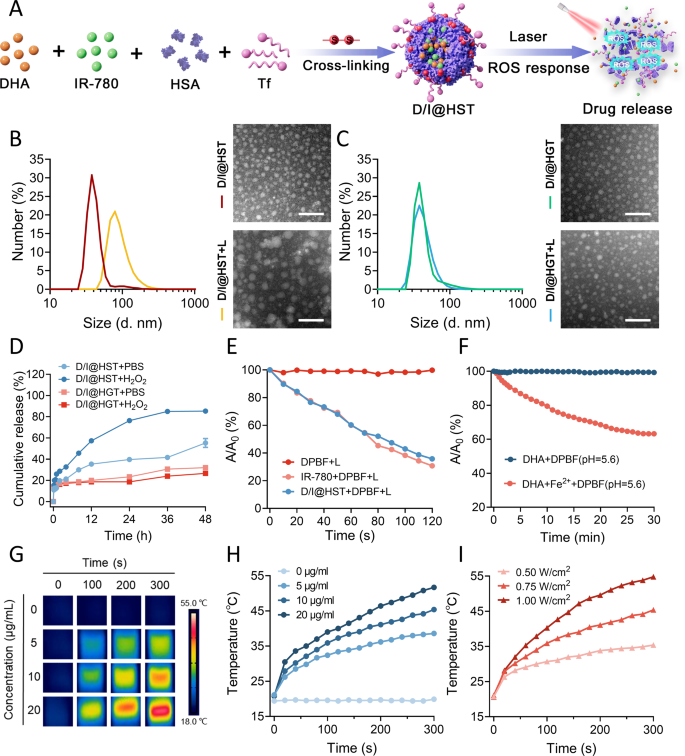
Synthesis and physicochemical characterization of D/I@HST NPs
The scheme for the synthesis of the D/I@HST NPs is proven in Fig. 1A. Briefly, DHA and IR-780 have been loaded onto HSA by means of electrostatic and hydrophobic interactions utilizing the ROS-responsive crosslinking agent PDDA. The nanoparticles floor was modified with Tf to kind D/I@HST NPs. The profitable technology of D/I@HST NPs was confirmed through ultraviolet-visible (UV-Vis) absorption spectroscopy (Fig. S1). The zeta potential of D/I@HST NPs was − 11.4 ± 1.4 mV. Furthermore, the particle dimension of D/I@HS NPs have been 37.63 ± 1.714 nm, whereas D/I@HST NPs have been 40.46 ± 8.206 nm, as measured by dynamic mild scattering (DLS), confirming the profitable loading of Tf within the D/I@HST NPs (Fig. S2). Transmission electron microscopy (TEM) revealed that D/I@HST NPs had a uniform spherical form (Fig. 1B). UV-Vis spectrophotometry revealed that the encapsulation effectivity and loading price of IR-780 within the D/I@HST NPs was 54.83% and three.98%, respectively (Fig. S3A and S3B). The encapsulation effectivity and loading price of DHA have been 93.65% and eight.32%, respectively, as decided by high-performance liquid chromatography (HPLC) (Fig. S3C).
To exhibit the ROS responsiveness of the nanomaterials, D/I@HGT NPs (non-ROS responsive NPs) have been used as controls. As glutaraldehyde is secure within the presence of ROS, D/I@HGT NPs ready utilizing glutaraldehyde as a crosslinking agent are usually not conscious of ROS. As proven in Fig. 1B, after 5 min of laser irradiation, the particle dimension of D/I@HST NPs modified from 40.46 ± 8.206 to 91.58 ± 35.11 nm (Fig. 1B). TEM confirmed the rise in D/I@HST NPs dimension and that their form was extra irregular. In distinction, no vital adjustments have been noticed within the D/I@HGT NPs after laser irradiation (Fig. 1C).
The cumulative launch price of IR-780 from the nanomaterials was examined in several launch media. The cumulative launch price of IR-780 from D/I@HST NPs at 48 h was 55.33% in phosphate-buffered saline (PBS) and 85.33% in 0.5 mM H2O2 (Fig. 1D). This is likely to be attributed to the ROS-reactivity of the crosslinker, leading to elevated drug launch. There was no distinction within the quantity of IR-780 launched from D/I@HGT NPs between PBS and H2O2 (26.67% and 32.00%, respectively).
A fluorescent probe, 1,3-diphenylisophenylfuran (DPBF), was used to evaluate the oxidative stress amplification properties of the D/I@HST NPs. This probe is extremely particular for 1O2 and causes the technology of 1,2-dibenzoylbenzene, lowering the absorbance at roughly 410 nm, which signifies 1O2 technology. DPBF confirmed no apparent lower in absorbance at 410 nm beneath laser irradiation, indicating that the probe was secure beneath irradiation (Fig. 1E). After 120 s of laser irradiation, the IR-780 absorbance at 410 nm considerably decreased. An analogous lower was noticed for D/I@HST NPs beneath laser irradiation, indicating that the ROS technology efficiency of IR-780 was not lowered when encapsulated in nanomaterials. Moreover, DHA and Fe2+ lowered the absorbance of the DPBF probe at pH 5.6 (Fig. 1F). This can be as a consequence of a Fenton-like response between DHA and Fe2+, inflicting the peroxide bridge of DHA to interrupt and produce carbon-centered oxygen radicals (Fig. S4). These outcomes exhibit the multi-ROS technology pathway induction impact of D/I@HST NPs.
IR-780 reveals photodynamic (PD) and photothermal (PT) results beneath near-infrared laser irradiation [40]. Thus, we validated the PT efficiency of IR-780 by thermal imaging. When uncovered to an 808-nm laser (energy density: 0.75 W/cm²), the temperature of the nanomaterial suspensions constantly elevated in a concentration-dependent method (Fig. 1G and H). Upon laser irradiation for 300 s, the temperature of 20 µg/mL IR-780 reached 51.7 ℃. Furthermore, the PT efficiency of the D/I@HST NPs was power-dependent (Fig. 1I). The temperature of the answer containing 10 µg/mL IR-780 reached 35.4 ℃ when irradiated at 0.50 W/cm² for 300 s. When the laser energy density was elevated to 1.00 W/cm², the temperature reached 54.8 °C, representing a rise of roughly 34 ℃. As well as, primarily based on the temperature curves of the nanomaterial suspensions throughout one laser irradiation and cooling cycle and the temperature-driven unfavourable pure logarithm relationship curve, the photothermal conversion effectivity (η) of D/I@HST NPs was 41.3% (Fig. S5). This impact brought about irreversible injury to the tumor cells throughout subsequent remedies.
The soundness of D/I@HST NPs in several media was additionally assessed. After seven days of storage in PBS, Roswell Park Memorial Institute (RPMI)-1640, and Dulbecco’s modified eagle medium (DMEM), no vital adjustments have been noticed within the particle dimension or polydispersity index (PDI) of D/I@HST NPs (Fig. S6A and S6B). Within the later stage of storage, the PDI of D/I@HST NPs in serum elevated, presumably as a consequence of serum protein aggregation over time. In the meantime, in H2O2, the particle dimension of D/I@HST NPs initially elevated earlier than lowering as a consequence of ROS responsiveness. These adjustments in D/I@HST NPs throughout the completely different media throughout storage have been confirmed by TEM. The D/I@HST NPs displayed good stability and ROS responsiveness (Fig. S6C). To evaluate the security of the nanomaterials, hemolysis assays have been carried out with D/I@HST NPs. Inside the therapeutic focus vary, the pink blood cell rupture price was < 5% (Fig. S7), demonstrating its excessive security stage.
Preparation and characterization of D/I@HST NPs. (A) Schematic diagram of D/I@HST NPs synthesis and ROS response. (B) DLS evaluation of the particle dimension distribution and consultant TEM photographs of D/I@HST NPs with and with out laser irradiation. Scale bar, 100 nm. (C) DLS evaluation of the particle dimension distribution and consultant TEM photographs of D/I@HGT NPs with and with out laser irradiation. Scale bar, 100 nm. (D) Cumulative launch price of IR-780 within the presence or absence of H2O2, n = 3. (E) DPBF-based detection of ROS technology induction by IR-780 beneath laser irradiation. (F) DPBF-based detection of ROS manufacturing from the response between DHA and Fe2+ at pH 5.6. (G) Thermal infrared imaging of D/I@HST NPs at completely different concentrations after laser irradiation for various intervals. (H) Temperature improve versus time plot beneath laser irradiation for various D/I@HST NPs concentrations. (I) Temperature improve versus time plot for D/I@HST NPs uncovered to completely different laser irradiation powers
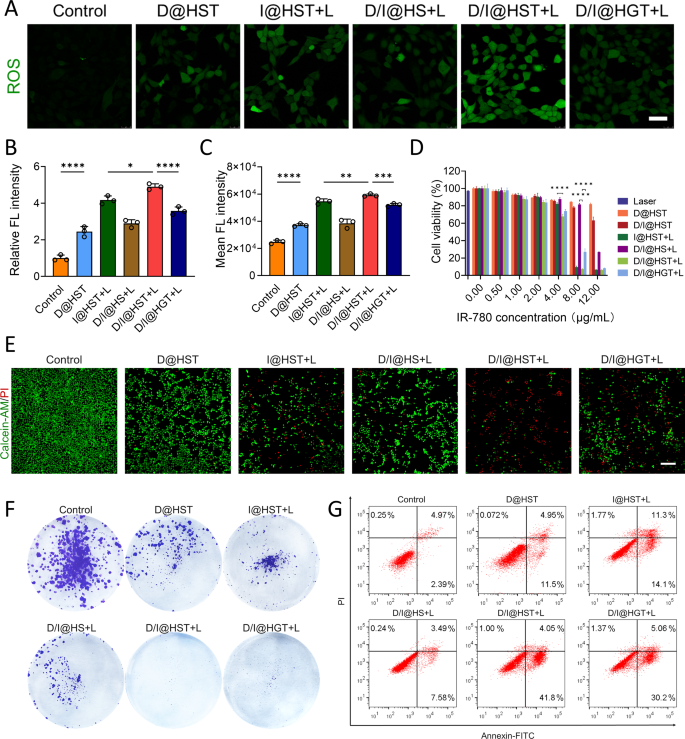
In vitro anti-tumor exercise of D/I@HST NPs
Earlier than verifying the anti-cancer exercise of nanomaterials, we confirmed their focusing on results. TfR is overexpressed in varied tumors, subsequently, Tf-modified nanomaterials can considerably improve their focusing on skill. As proven in Fig. S8A, when the Tf-modified D/I@HST NPs have been co-cultured with 4T1 cells, their accumulation progressively elevated over time. The buildup of D/I@HST in tumor cells was considerably greater than that of Tf-unmodified D/I@HS (Fig. S8B-D). To confirm the position of Tf in focusing on tumor cells, 4T1 cells have been pretreated with 20 µM Tf for 1 h earlier than drug therapy. The IR-780 sign was considerably lowered in Tf-pretreated cells. These outcomes point out that the focusing on impact of Tf-modified D/I@HST NPs was considerably enhanced, bettering their accumulation in tumor cells.
To validate the impact of D/I@HST NPs on ROS technology on the mobile stage, confocal laser scanning microscopy (CLSM) was employed to detect diacetyldichlorofluorescein diacetate (DCFH-DA) in 4T1 cells after therapy with the assorted nanomaterial preparations. Cells handled with D@HST NPs displayed a pronounced inexperienced fluorescence sign in contrast with the untreated group (Fig. 2A and B). This was attributed to the focusing on impact of the modified Tf on the D@HST NPs and Fe3+ discount to Fe2+ by ferric reductase within the acidic tumor cell setting, which reacted with DHA to generate ROS. Concurrently, 4T1 cells handled with I@HST NPs generated a big quantity of ROS after laser irradiation at 808 nm (1.0 W/cm2) for five min. A fluorescence microplate reader was used to verify related ROS technology capabilities throughout the completely different therapy teams (Fig. 2C). D/I@HST NPs induced probably the most ROS technology because of the simultaneous motion of the 2 pathways of ROS technology. In the meantime, the suboptimal ROS technology within the D/I@HGT NPs might be attributed to the shortage of ROS responsiveness of the crosslinking agent, stopping the whole launch of the drug.
CCK-8 evaluation was used to confirm the in vitro synergistic anti-tumor results of the D/I@HST NPs after internalization. Laser irradiation at 808 nm (1.0 W/cm2) for five min had no cytotoxic impact on 4T1 cells (Fig. 2D). As well as, the cytotoxic impact of the D@HST NPs on the cells was comparatively weak at equal concentrations of IR-780. At 8 µg/mL IR-780, I@HST NPs mixed with laser irradiation lowered cell survival to 9.56%. Therefore, the phototherapeutic impact of IR-780 performed a important position in therapy. Certainly, marked cytotoxicity was noticed in all teams handled with D/I@HST NPs and laser irradiation in any respect concentrations, attributed to multi-pathway oxidative stress amplification and PTT. These outcomes have been supported by the dwell/lifeless staining, which additional demonstrated the robust in vitro anti-tumor impact of the D/I@HST NPs. The variety of lifeless cells considerably elevated after therapy with D/I@HST NPs and laser irradiation (Fig. 2E). The proliferation of 4T1 cells after the completely different remedies was evaluated utilizing the plate cloning technique. Crystal violet staining revealed that the proliferation price of the 4T1 cells was considerably decrease within the D/I@HST + L group (Fig. 2F and S9A). Subsequent evaluation of 4T1 cell apoptosis utilizing an Annexin FITC/PI dual-staining cell apoptosis detection package revealed an apoptosis price of 45.85% following therapy with D/I@HST + L at an IR-780 focus of 6 µg/mL (Fig. 2G and S9B). General, these outcomes confirmed that the D/I@HST NPs exhibited passable anti-tumor results in vitro.
ROS manufacturing and in vitro anti-tumor results of D/I@HST NPs in 4T1 cells. (A) Consultant CLSM photographs of ROS manufacturing in 4T1 cells handled with completely different NPs at an equal focus of 1 µg/mL IR-780 (+ L, with laser irradiation). Scale bar, 50 μm. (B) Statistical evaluation of (A) utilizing ImageJ software program; n = 3. (C) Intracellular ROS ranges assessed utilizing the DCFH-DA assay, n = 3. (D) CCK-8 assay evaluation of 4T1 cell viability following laser irradiation, D@HST, D/I@HST, I@HST + L, D/I@HS + L, D/I@HST + L, or D/I@HGT + L (+ L, with laser irradiation), n = 3. (E) Fluorescent photographs of dwell/lifeless 4T1 cell staining following therapy with completely different nanomaterials; Scale bar, 200 μm. (F) Colony formation evaluation of 4T1 cells one week after therapy with completely different nanomaterials. (G) Circulation cytometry evaluation of 4T1 cell apoptosis induced by completely different nanomaterials utilizing Annexin V-FITC/PI staining. Information are introduced as imply ± SD. Statistical evaluation was carried out utilizing one-way ANOVA adopted by Tukey’s check for (B) and (C). *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001
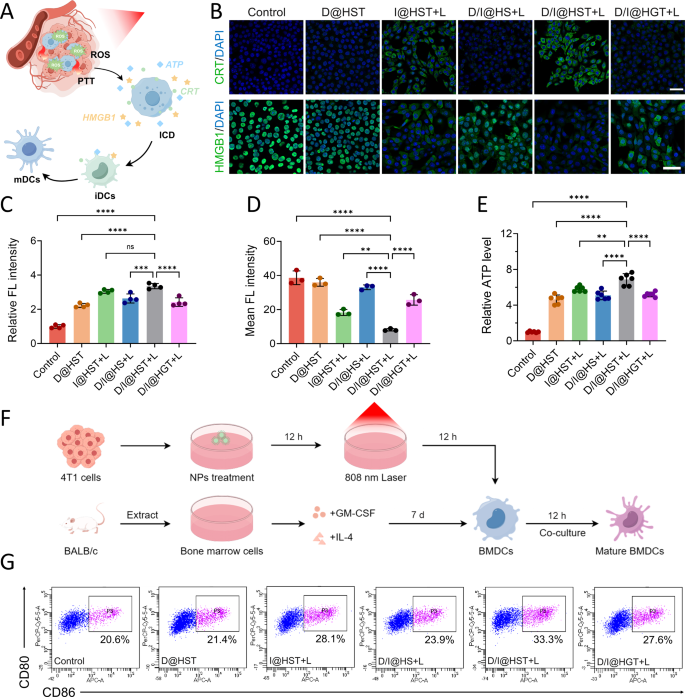
In vitro immune activation induced by D/I@HST NPs
Detection indicators associated to ICD have been found and confirmed, primarily by three approaches. First, calreticulin (CRT) on the floor of dying cells serves as a potent “eat me” sign that stimulates recognition by dendritic cells (DCs). Second, the discharge of ATP is a “discover me” sign that recruits DCs to the tumor website. Third, high-mobility group field 1 (HMGB1) on tumor cells promotes secure binding with DCs, inducing particular anti-tumor immunity within the physique [41,42,43]. Accordingly, we validated the induction of ICD by D/I@HST NPs in 4T1 cells (Fig. 3A). As proven in Fig. 3B and C, and S10, D@HST NPs displayed a sure skill to generate ROS in tumor cells and promote endoplasmic reticulum stress, resulting in a weak impact on selling CRT publicity. The CRT fluorescence sign of the D/I@HST NP therapy group was the strongest, confirming that multi-pathway oxidative stress and PTT synergistically promoted ICD. Furthermore, CLSM revealed that cells handled with D/I@HST NPs confirmed considerably enhanced translocation of HMGB1 from the nucleus to the extracellular house (Fig. 3B and D, and S11). Detection of ATP ranges within the cell tradition media additional revealed that D/I@HST NPs strongly stimulated ATP launch from dying cells (Fig. 3E). Collectively, these findings recommend that D/I@HST NPs mixed with laser therapy promote ICD-related indicators, which can enhance the anti-tumor immune response.
To additional validate the results of D/I@HST NPs on DCs maturation, we extracted bone marrow-derived dendritic cells (BMDCs) from the femur and tibia of BALB/c mice. Subsequently, the tradition medium of 4T1 cells handled with the nanomaterials was cocultured with BMDCs, and circulate cytometry was used to evaluate the flexibility of the nanomaterials to stimulate BMDCs maturation (Fig. 3F). Below the therapy of 808 nm laser, D/I@HST NPs produced PTT impact and multi-path oxidative stress amplification, successfully induced the incidence of ICD in 4T1 cells, thereby considerably selling BMDCs maturation (Fig. 3G).
D/I@HST NP–induced immunogenic cell demise in 4T1 cells. (A) Schematic diagram of nanomaterial-induced immunogenic cell demise of 4T1 cells and selling the maturation of immature DCs beneath laser irradiation. (B) Consultant CLSM photographs of CRT publicity on the floor of 4T1 cells and intracellular HMGB1 in 4T1 cells handled with tradition medium, D@HST, I@HST + L, D/I@HS + L, D/I@HST + L, or D/I@HGT + L (+ L, with laser irradiation). Scale bar, 50 μm. (C) CRT ranges in (B) obtained utilizing ImageJ software program, n = 4. (D) Intracellular HMGB1 ranges in (B) decided utilizing ImageJ software program, n = 3. (E) Mobile ATP efflux ranges in several therapy teams, n = 6. (F) Schematic diagram of BMDCs extraction and maturation. (G) Consultant circulate cytometry plot of BMDCs maturation after co-incubation with 4T1 cells and completely different remedies. Information are introduced because the means ± SD. Statistical evaluation was carried out utilizing one-way ANOVA adopted by Tukey’s check for (B), (C), and (E). Significance is outlined as ns, no significance, **P < 0.01, ***P < 0.001, and ****P < 0.0001
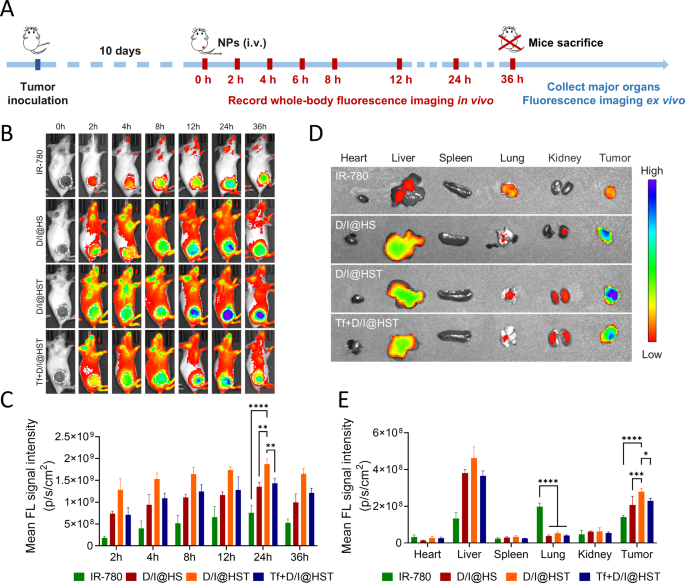
In vivo D/I@HST NP focusing on and distribution
The distribution of Tf-modified nanomaterials was evaluated in vivo utilizing a 4T1 tumor-bearing mouse mannequin. When tumor quantity reached 200 mm3, the mice have been randomly divided into 4 teams: IR-780, D/I@HS, D/I@HST, and Tf + D/I@HST. Free IR-780, D/I@HS, D/I@HST, or Tf + D/I@HST (Tf = 50 µM) have been injected into the tail veins of mice. The focus of the IR-780 imaging agent was 80 µg/kg. Dwell imaging of small animals was carried out, and 36 h after drug administration, the main organs and tumors have been acquired for ex vivo fluorescence imaging (Fig. 4A). IR-780 is a lipophilic cationic NIR dye that may goal tumors however is quickly metabolized (Fig. 4B). In the meantime, encapsulation of IR-780 in Tf-modified nanomaterials considerably enhanced its accumulation on the tumor website. In distinction, the fluorescence sign depth on the tumor website decreased considerably within the Tf pretreatment group. This was possible because of the TfRs expressed on tumor cells changing into saturated with the exogenous Tf, inflicting aggressive antagonism with the Tf on the D/I@HST NPs. The D/I@HST NPs abundance peaked 24 h after administration in vivo (Fig. 4C). Ex vivo fluorescence imaging revealed that the D/I@HST NPs encapsulating IR-780 have been metabolized within the liver, whereas free IR-780 was characterised by non-specific uptake and tended to build up within the lungs, leading to poor selectivity and low drug utilization (Fig. 4D and E). These findings have been in line with outcomes from a earlier examine [44]. At 36 h after administration, D/I@HST NPs nonetheless confirmed robust fluorescence, indicating that Tf modification of D/I@HST NPs considerably enhanced their skill to build up on the tumor website.
In vivo focusing on and distribution of D/I@HST NPs. (A) Experimental define of fluorescence imaging in vivo. (B) In vivo whole-body fluorescence imaging of mice at varied time factors after the injection of the completely different nanomaterials. (C) Semi-quantitative evaluation of fluorescence alerts of the tumor space for 36 h after injection, n = 3. (D) Ex vivo fluorescence photographs of tumors and main organs 36 h after injection. (E) Quantitative biodistribution evaluation of D/I@HST NPs in main organs of mice primarily based on ex vivo fluorescence imaging in (D), n = 3. Information are introduced because the imply ± SD. Statistical evaluation was carried out utilizing two-way ANOVA adopted by Tukey’s check for (C) and (E). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001
In vivo synergistic therapeutic results of D/I@HST NPs
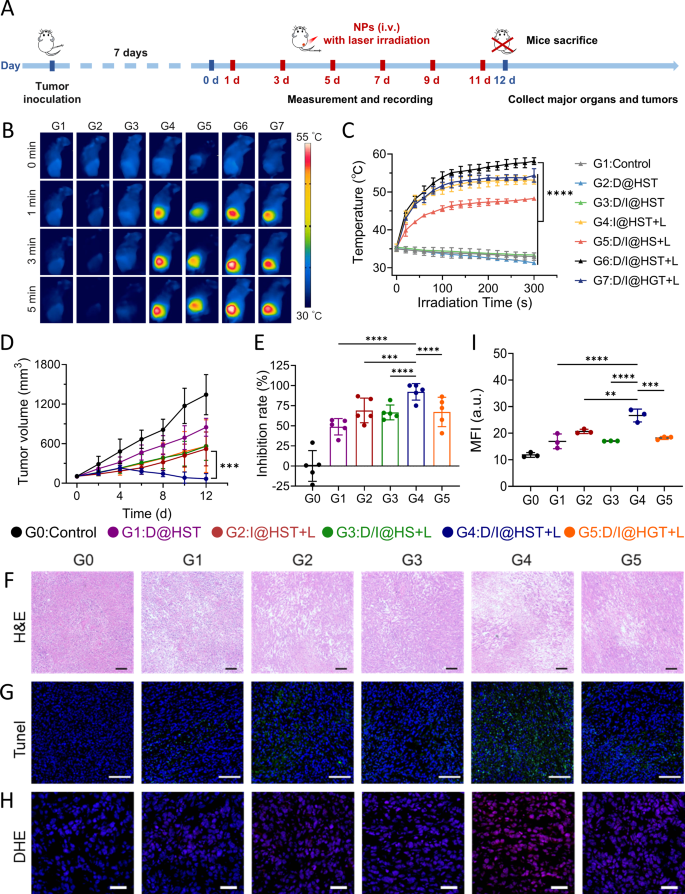
A cell line-derived xenograft (CDX) mannequin was established in BALB/c mice to guage the anti-tumor results of D/I@HST NPs. When tumor volumes reached 100 mm3, the mice have been divided into six teams: PBS (Management), D@HST, I@HST, D/I@HS, D/I@HST, and D/I@HGT. The mice in every group have been administered the respective remedies (Fig. 5A). The mice administered the nanomaterials containing the IR-780 photosensitizer have been subjected to irradiation with an 808-nm laser (1.0 W/cm2) irradiation for five min, throughout which the temperature improve on the tumor website was detected utilizing near-infrared thermography. Within the mice not subjected to laser irradiation, a lower in temperature was detected on the tumor website (Fig. 5B). This may occasionally have been because of the tendency of mice to lose warmth beneath anesthesia. The insufficient heating impact within the D/I@HS group was attributed to the restricted accumulation of the nanomaterials on the tumor website, leading to an inadequate quantity of IR-780 being launched (Fig. 5C). Notably, the temperature of the tumor website within the D/I@HST group elevated to 58 °C after 5 min following laser irradiation, indicating a passable PTT impact.
Through the therapy course of, we monitored the tumor dimension and evaluated the therapeutic results of various nanomaterials. Nanomaterial teams confirmed stronger tumor growth-inhibitory results than the PBS group (Fig. 5D and E, and S12). The tumor growth-inhibition price of the D@HST group was 48.75%, as a consequence of breakage of the DHA peroxide bridge by Fe2+, resulting in ROS manufacturing and enhanced chemotherapeutic results. Because of the robust phototherapeutic skill of the I@HST + L therapy, the tumor inhibition price reached 69.04%. Nonetheless, probably the most vital therapy impact was noticed within the D/I@HST + L group, with a lower in common tumor quantity being noticed after 4 days of therapy. On the finish of the therapy protocol, two tumors disappeared, with the tumor inhibition price reaching 90.97%. These findings verify that D/I@HST NPs enhanced the extent of oxidative stress upon laser irradiation, selling the synergistic inhibition of tumor progress. Hematoxylin and eosin (H&E) staining of mouse tumor sections was used to evaluate necrosis at tumor websites. The tumor cells within the D/I@HST + L group exhibited the very best stage of necrosis (Fig. 5F). Moreover, terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) immunofluorescence staining was carried out to evaluate tumor cell apoptosis and exhibit the therapeutic potential of the nanomaterials. The biggest proportion of apoptotic cells was noticed in D/I@HST + L group, indicating that synergistic remedy successfully promoted tumor cell apoptosis (Fig. 5G). Dihydroethidium (DHE) staining confirmed that D/I@HST NPs have been able to upregulating ROS manufacturing following laser irradiation, even in vivo (Fig. 5H and I).
The in vivo biosafety of the nanomaterial was assessed. Through the tumor therapy course of, no vital weight reduction was detected within the mice (Fig. S12D). Moreover, blood was collected on the finish of therapy for biochemical evaluation. Nonetheless, no vital variations have been noticed within the liver or kidney features of the mice from the completely different therapy teams (Fig. S13). Furthermore, the analyses of H&E-stained sections of main organs revealed no outstanding abnormalities (Fig. S14). These outcomes demonstrated that this synergistic therapy technique is related to a good security profile in mice.
In vivo photothermal conversion impact and anti-tumor impact of D/I@HST NPs. (A) Schematic define of in vivo anti-tumor experiments. (B) Consultant thermographic photographs of tumor websites in mice irradiated with laser for various durations following injection with completely different nanomaterials. (C) Native temperature of tumors irradiated with an 808 nm laser for five min. (D) Tumor progress kinetics of common tumor volumes from mice handled with PBS, D@HST, I@HST + L, D/I@HS + L, D/I@HST + L, or D/I@HGT + L (+ L, with laser irradiation), n = 5. (E) Tumor inhibition price throughout the completely different therapy teams. (F) Consultant photomicrographs of tumor sections of the mice from the completely different therapy teams obtained after H&E staining. Scale bar, 100 μm. (G) TUNEL staining for apoptosis in tumors extracted from the mice subjected to the completely different remedies. Scale bar, 100 μm. (H) DHE immunofluorescence staining for the evaluation of ROS accumulation in tumor sections of mice from the completely different therapy teams. Scale bar, 50 μm. (I) Statistical evaluation of the imply fluorescence depth of DHE in (H), n = 3. Information are introduced because the imply ± SD. Statistical evaluation was carried out utilizing two-way ANOVA adopted by Tukey’s check for (C) and (D) and one-way ANOVA adopted by Tukey’s check for (E) and (I). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001
In vivo immunomodulation of D/I@HST NPs
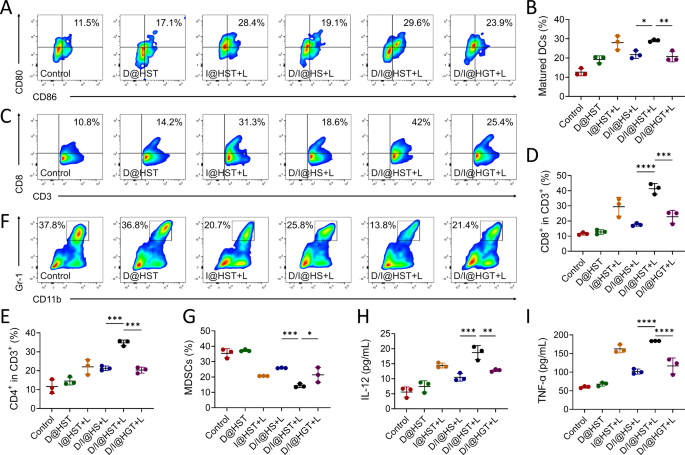
On the mobile stage, we validated that that D/I@HST NPs might successfully induce ICD in tumor cells after laser irradiation and trigger the discharge of tumor-associated antigens and DAMPs, thereby selling the BMDCs maturation. To guage the immune activation induced by ICD in vivo, we obtained handled tumors and ready single-cell suspensions. Circulation cytometry was used to check the DCs maturation within the handled tumors. A consultant gating technique utilized within the circulate cytometry analyses is introduced in Fig. S15. Per the noticed maturation of BMDCs in vitro, the I@HST and D/I@HST teams demonstrated enhanced DCs maturation, with the proportions of mature DCs reaching 28.4% and 29.6%, respectively (Fig. 6A and B). This was attributed to the robust immune activation induced by ICD owing to IR-780-mediated phototherapy. Mature DCs are acknowledged for his or her position in presenting tumor-associated antigens, which promotes T-cell infiltration into tumors. Therefore, CD8+ and CD4+ T cell infiltration into the tumors was investigated. The mice from the D/I@HST + L group exhibited stronger immunostimulatory results within the immunosuppressive TME than these within the different teams, additionally exhibiting enhanced lymphocyte infiltration into the tumors (Fig. 6C–E and S16). Furthermore, the mice from the D/I@HST + L group exhibited the bottom ranges of MDSCs within the TME, confirming that MDSCs had the weakest immunosuppressive impact on the anti-tumor efficacy of CTLs (Fig. 6F and G). These findings additional confirmed that D/I@HST NPs can cut back tumor-associated immune suppression and reverse the formation of the “chilly” immune TME. Elevated ranges of interleukin (IL)-12 and tumor necrosis factor-alpha (TNF-α) are markers of immune activation in TME. Therefore, serum IL-12 and TNF-α ranges have been assessed through enzyme-linked immunosorbent assays (ELISAs). The D/I@HST + L group exhibited the very best ranges of IL-12 and TNF-α (Fig. 6H and I). These outcomes recommend that D/I@HST NPs induce a tumor cell-killing impact in vivo, and launch tumor-associated antigens to advertise DCs maturation, improve T cell infiltration, and reverse the immunosuppressive microenvironment.
Immune enhancement induced by completely different remedies in mice. (A–G) Consultant circulate cytometry plots and proportional statistics indicating the proportions of (A, B) mature DCs in tumors, (C, D) CD8+ T cells in CD3+ T cells, (E) CD4+ T cells in CD3+ T cells, and (F, G) MDSCs of 4T1 tumor-bearing mice following varied remedies, n = 3. (H, I) Ranges of serum (H) IL-12 and (I) TNF-α following varied interventions, n = 3. Information are introduced because the imply ± SD. Statistical evaluation was carried out utilizing one-way ANOVA adopted by Tukey’s check for (B), (D), (E), (G), (H), and (I). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001
D/I@HST NPs mixed with PD-L1 inhibitors inhibit main and distal tumor progress
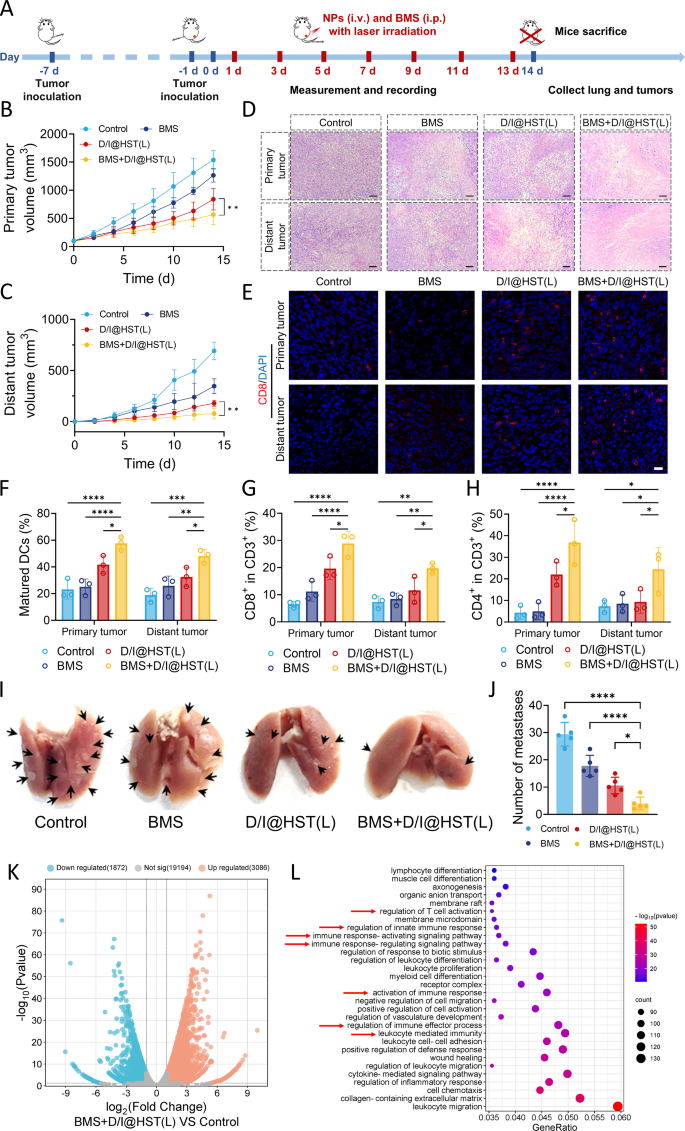
Immunotherapy, represented by immune checkpoint inhibitors, has led to vital progress in most cancers therapy [45]. Tumor cells make the most of sure immune checkpoints as the primary mechanism for immune resistance and evasion, significantly by programming cytotoxic T lymphocytes (CTLs) to focus on tumor antigens [46]. At present, probably the most studied immune checkpoints embrace programmed cell demise protein 1(PD-1) and its ligand, programmed cell demise 1 ligand 1 (PD-L1) [47,48,49]. Many tumor cells specific PD-L1, which binds to PD-1 on T cells, activating immune checkpoints, inhibiting T cell activation, and inducing T cell apoptosis [50, 51]. Because the activation of immune checkpoints is primarily triggered by the binding of receptors and ligands, their binding might be blocked by inhibitors [52]. Due to this fact, we lowered the dose of D/I@HST NPs (IR-780 from 500 to 250 µg/kg) and mixed them with a PD-L1 inhibitor (BMS) to discover the inhibitory results on the expansion of bilateral tumors in mice (Fig. 7A). The expansion of main tumors in mice handled with low-dose D/I@HST NPs after 808-nm laser irradiation was barely delayed in contrast with that within the BMS group ((Fig. 7B, S17A, and S17B). Notably, the mixture remedy group demonstrated a passable therapeutic impact, with marked tumor inhibition. The identical pattern was noticed with regard to the expansion of distal tumors. The BMS + D/I@HST(L) group exhibited probably the most vital anti-tumor impact, with one distal tumor disappearing after therapy (Fig. 7C, S17C, and S17D). H&E staining additional substantiated the efficient tumor suppression by the BMS + D/I@HST(L) mixture remedy. This can be as a consequence of BMS blocking PD-L1 and PD-1 binding and selling T-cell infiltration into distal tumors. The cell densities of the first and distal tumors confirmed a considerable discount after mixture remedy, with a higher stage of necrosis (Fig. 7D). Moreover, mixed therapy didn’t considerably affect mouse weight, demonstrating its relative biocompatibility (Fig. S17E).
To visualise CD8+ T cell infiltration into the tumor website, immunofluorescence staining of the tumor sections was carried out. Markedly greater numbers of CD8+ T cells infiltrated the tumors within the BMS + D/I@HST(L) group than within the BMS or D/I@HST(L) teams (Fig. 7E). Moreover, circulate cytometry was used to investigate DCs maturation, T cell infiltration, and MDSCs abundance on the tumor website. Mixture remedy considerably enhanced the DCs maturation within the main tumor by 58% (Fig. 7F and S18). Notably, the maturation impact of DCs in distal tumors after mixture therapy was considerably higher than that after therapy with D/I@HST(L) or BMS alone. T cell infiltration within the bilateral tumors was considerably enhanced after mixture therapy (Fig. 7G and H, and S19), suggesting that BMS successfully blocked the binding of PD-L1 and PD-1, resulting in a considerable enhancement of phototherapy-induced immune activation by D/I@HST NPs. Furthermore, mixture remedy successfully lowered the variety of MDSCs within the tumors and reversed the formation of an immunosuppressive TME (Fig. S20). Evaluation of the lungs obtained from mice that had undergone mixture remedy revealed efficient suppression of lung metastasis, with a mean variety of solely 4 metastatic nodes, owing to the highly effective immune activation impact, whereas lungs of the untreated group had a mean of almost 30 tumor metastatic nodes (Fig. 7I and J, and S21).
Subsequent, we studied the immune mechanism of BMS mixed with D/I @HST (L) in inhibiting tumor progress. After therapy, a part of the tumor tissues was excised for transcriptome sequencing. As proven in Fig. 7Ok and 4958 genes have been differentially expressed between the BMS + D/I@HST(L) and management teams, with 3086 upregulated and 1872 downregulated genes. Purposeful enrichment evaluation of the upregulated genes was carried out. As proven in Fig. 7L, the vertical axis represents completely different pathways, and the horizontal axis represents the gene ratio (the proportion of genes enriched within the goal pathway to the full genes). The dimensions of the dots represents the variety of genes, and the colour represents the scale of the distinction. Because the dots darkened, enrichment grew to become outstanding. Gene Ontology (GO) purposeful enrichment evaluation confirmed that the upregulated genes within the BMS + D/I@HST(L) group have been concerned in regulating T cells activation, modulating innate immune response, and regulating the immune response. These outcomes indicated that BMS mixed with D/I@HST(L) successfully activated the immune response, which is in line with the circulate cytometry outcomes.
Mixed utility of D/I@HST NPs and PD-LI inhibitors exerts potent tumor suppression and immune activation results. (A) Schematic diagram of bilateral tumor development and mixed remedy. (B, C) Development kinetics curves of (B) main and (C) distant tumors in mice handled with PBS, BMS, D/I@HST(L), or BMS + D/I@HST(L) (“L”, with laser irradiation), n = 5. (D) Consultant photographs of H&E-stained tumor sections on either side of mice of various therapy teams. Scale bar, 100 μm. (E) Consultant photographs of CD8+ T cell immunofluorescence staining on tumor sections from either side of mice of various therapy teams. Scale bar, 20 μm. (F–H) The chances of mDCs (F), CD8+ T (G) and CD4+ T (H) cell populations in tumors following varied remedies, n = 3. (I) Consultant photographs of lungs obtained from mice in several therapy teams, arrows point out lung metastasis node. (J) Quantification of lung metastasis nodes in several teams, n = 5. (Ok) Volcanic map reveals differentially expressed genes between the BMS + D/I@HST(L) and management teams. (L) GO purposeful enrichment evaluation of genes considerably upregulated within the BMS + D/I@HST(L) vs. management teams. Information are introduced as imply ± SD. Statistical evaluation is carried out utilizing two-way ANOVA adopted by Tukey’s check for (B) and (C) and one-way ANOVA adopted by Tukey’s check for (F–H) and (J). Significance is outlined as ns, no significance, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001