Synthesis and characterization of DMUP
Determine 2a illustrates the synthesis means of the NIR-IIb fluorescence imaging multifunctional nanozyme platform (DMUP). First, a thermal decomposition methodology was utilized to synthesize 980 nm NIR-responsive DCNPs with a core-shell construction (Fig. 2b and c, and S1) [38]. The ensuing DCNPs have been homogeneous monodispersed hexagonal core-shell nanoparticles with a particle dimension of about 35 nm. To extend the hydrophilicity and facilitate the next purposeful steps, a layer of mSiO2 roughly 30 nm thick was grown on the floor of the DCNPs, leading to DCMS (Fig. 2d and S2). Subsequently, the DCMS floor was functionalized to kind DCMS-COSR. Then, Mn nanozyme was precipitated into the pores of DCMS-COSR, adopted by the grafting of UBI29 − 41 onto its floor and the loading of Pae into remaining pores, in the end forming the resultant advanced known as DMUP. As proven in Fig. 1, the transmission electron microscopy (TEM) picture revealed that the Mn nanozyme nanoparticles appeared on the floor of the DCMS in a attribute ribbon-like construction [27, 30]. Furthermore, the scanning electron microscopy (SEM) outcomes confirmed a raised form on the pores (Fig. S3). The profitable preparation of DMUP was additionally proved by the fundamental mapping outcomes (Fig. 2f).
Synthesis and characterization of DMUP. (a) A schematic diagram displaying the synthesis of DMUP. The TEM photographs of (b) DCNPs core, (c) DCNPs, (d) DCMS, and (e) DMUP. (f) Elemental mapping evaluation of DMUP. (g) Zeta potential modifications of the DM-COSR earlier than and after being floor grafted of UBI29 − 41. (h) XRD patterns of DCNPs, DCMS, and DMUP. (i) XPS power spectrum for DMUP. (j) The fitted peak spectrum of the Mn factor after deconvolution. (ok) FTIR spectra of Core, DCNPs, DCMS, DCMS-COOH, DCMS-COSR, and DMU. (l) NIR-IIb fluorescence broad spectra of the DCNPs core, DCNPs and DCMS. (m) NIR-IIb fluorescence spectrum of DCNPs beneath 980 nm excitation and NIR-IIb absorption spectrum of DMUP. (n) Fluorescence spectra of DMUP synthesized with completely different concentrations of Mn doping within the NIR-IIb band. Information are means ± s.d. (n ≥ 3)
The grafting on the mSiO2 floor started with modifying the N-terminus of UBI29 − 41 with cysteine (Fig. S4). Then, the DCMS-Mn (denoted as DM) floor reacted by way of thioesterification to kind DM-COSR (Fig. S5) [39]. Lastly, as proven in Determine S6, the peptide was covalently connected by way of native chemical ligation (NCL) to kind DCNP@mSiO2-Mn-UBI29 − 41 (denoted as DMU) [35]. We additionally complemented the covalent docking simulations, with Fig. S7 displaying that the thiol and amine teams of cysteine in UBI29 − 41 may kind covalent bonds with the carbonyl group of DM-COSR, leading to a steady amide bond with a docking rating of − 9.226 kcal/mol (a docking rating under − 5 kcal/mol signifies bond stability). The profitable synthesis of DMU was confirmed by evaluating the modifications in zeta potential and pH throughout the grafting course of (Fig. 2g and S8). Fig. 2h exhibits the X-ray diffraction (XRD) sample of the system. The diffraction peaks might be attributed to the hexagonal part crystalline type of β-NaYF4 (JCPDS No. 00-016-0334). Moreover, as for the mSiO2 of DCMS, a broad absorption band of amorphous SiO2 might be noticed at 2θ = 22°. As well as, the outcomes of Fig. 2h confirmed attribute peaks according to the ZIF-8 crystal part (JCPDS No. 00-062-1030), and matched with diffraction peaks of MnO2 (JCPDS No. 00-072-1982). This supported the formation of metal-organic frameworks in DMUP pores and the speculation that MnO2 served because the nanozyme’s lively element. And ZIF-8 metal-organic framework contributed to acid-responsive performance. The ultraviolet-visible spectroscopy (UV-vis) spectrum of DM-COSR revealed the attribute broad absorption peak after Mn nanozyme precipitation (Fig. S5). Furthermore, the X-ray photoelectron spectroscopy (XPS) becoming outcomes revealed the Mn(IV) oxidation state within the DMUP (Fig. 2i and j). It was proven that the profitable precipitation of Mn nanozyme nanoparticles demonstrated the potential efficiency of tetravalent manganese antioxidant nanozyme [40]. In the meantime, fourier rework infrared (FTIR) was utilized to detect the purposeful teams (Fig. 2ok). The FTIR spectra revealed C − C and C − H peaks at 2900 cm− 1, which have been attribute of the DCNPs [38]. The C = O stretching vibration peaks between 1700 and 1725 cm− 1, and the enhancement of the O − H bonds between 2500 and 3300 cm− 1, demonstrated the profitable modification of the − COOH (DCMS-COOH). Furthermore, the attribute peaks of the aryl ring at 750–800 cm− 1 indicated the success of thioesterification (DCMS-COSR) [39].
The fluorescence properties throughout the synthesis of DMUP have been characterised as proven in Fig. 2l. DCMS exhibited a powerful emission peak within the 1500–1600 nm NIR-IIb fluorescence band beneath 980 nm excitation. The broad absorption of Mn nanozyme within the NIR band (Fig. 2m) led to FRET motion together with the precipitation of Mn nanozyme within the pore. As proven in Fig. 2n, the NIR-IIb fluorescence of DMUP was progressively quenched. The ultimate successfully quenched KMnO4 synthesized focus was estimated to be 62.5 µg mL− 1. The profitable loading of Pae was illustrated in Fig. S9 and S10. The XPS spectrum and inductively coupled plasma-mass spectrometry (ICP-MS) outcomes of DMUP revealed the presence of Er, Yb, Lu, and Si components in DMUP, additional confirming the profitable loading of every element in DMUP (Fig. S11–S15).
Evaluation of physicochemical properties and biosafety of DMUP
ROS are byproducts of regular metabolism, sometimes neutralized by the antioxidant system of physique beneath regular physiological situations. Throughout the development of periodontitis, the immune system turns into hyperactive because of the improve in anaerobic pathogenic micro organism. This course of will increase the quantity of ROS within the periodontal tissues, which causes an imbalance between ROS and the antioxidant system, leading to a poisonous response, thereby exacerbating periodontitis [41]. In the meantime, with the buildup of plaque, the periodontal microenvironment shifts from a normoxic to a hypoxic part. The resultant deep hypoxic setting additional exacerbates the colonization of pathogenic anaerobic micro organism [42, 43]. Due to this fact, so as to modulate the modifications within the periodontitis microenvironment, this examine verified the acid response, antioxidant system launch, CAT-like, SOD-like, and catalytic antioxidant nano-enzymatic properties of DMUP.
Determine 2a exhibits the involvement of reactive oxygen species in mobile respiration. First, O2 gained an electron to kind ·O2−, which was then transformed to H2O2 by SOD. Subsequent, CAT decomposed H2O2 to H2O and O2 [28]. Throughout the anti-inflammation course of, the surplus ROS is scavenged to provide O2, which alleviates the hypoxic setting and inhibits the anaerobic micro organism [26]. The ICP-MS and UV-vis spectroscopy (Fig. S16 and S17) outcomes revealed that Mn oxides and Pae launch elevated beneath acidic situations, offering the premise for a cascade catalytic response to extra ROS. Then, Fig. 2b demonstrated that DMUP may get better NIR-II fluorescence in acidic situations because of the elimination of Mn oxides from the pores, which eradicated FRET results [30]. Electron spin resonance (ESR) was utilized to substantiate that DMUP exhibited CAT-like and SOD-like actions by scavenging ·OH, ·O2− and H2O2. Determine 2c–e confirmed that DMUP scavenges important quantities of ·OH, ·O2− and H2O2. Moreover, this examine additionally addressed the difficulty of hypoxia in periodontal pockets. The H2O2 produced by the hyperactive immune response in infected tissues and the periodontal pocket bacterial biofilm throughout metabolism aggravates tissue harm [44]. Mn nanozyme incorporation offered DMUP with a powerful skill to decompose H2O2 into O2 (Fig. S18), which was extraordinarily important within the therapy of periodontal illnesses. Ample O2 manufacturing successfully alleviated periodontal pocket hypoxia, improved the hypoxic setting [26], and inhibited the proliferation of anaerobic pathogenic micro organism. As proven in Fig. 2f, DMUP may catalyze O2 manufacturing in several concentrations of H2O2 answer.
Inspired by the superb ROS scavenging and catalytic H2O2 O2-producing skill of DMUP in vitro, we subsequent examined these properties in cells. First, the impact of DMUP on the proliferation of the macrophage RAW264.7 cells, an necessary immune line of protection, was evaluated. As proven in Fig. S19–S22, the outcomes of cell counting kit-8 (CCK-8), cell reside/useless staining, and hemolysis take a look at indicated that DMUP at a focus of 200 µg mL− 1 was thought-about secure and utilized in subsequent cell experiments. Subsequently, bacterial LPS was chosen to induce an inflammatory mannequin of endogenous oxidative stress in RAW264.7 cells [34]. The ROS probe DCFH-DA was utilized to find out the antioxidant impact of DMUP. In contrast with the management (Con) group, the LPS group considerably promoted ROS manufacturing with robust fluorescence (Fig. 2g and h). In distinction, there was a fluorescence quenching within the DMUP group, indicating a discount within the intracellular ROS degree. This clearly prompt that DMUP decreased endogenous oxidative stress. Subsequent, the intracellular O2 manufacturing capability of DMUP in RAW264.7 was investigated by tris (4,7-diphenyl-1,10-phenanthroline) ruthenium (II) dichloride ([Ru(dpp)3]Cl2), a luminescent probe generally used for O2 identification and quantification (Fig. 2i and j). In distinction to the Con group, the amplification of pink luminescence indicated a lower within the O2 content material of the LPS-induced inflammatory cells. After incubating with DMUP for two h, the attenuation of pink luminescence confirmed O2 manufacturing (Fig. S23). This end result demonstrated that DMUP had intracellular skill to catalyze O2 manufacturing. Due to this fact, the catalytic decomposition of ROS may cut back irritation by regulating the redox imbalance and hypoxic state on the inflammatory website.
Physicochemical properties and biosafety of DMUP. (a) Antioxidant and O2-producing antimicrobial precept of DMUP. (b) NIR-II fluorescence spectra of DMU, DMUP, and DMUP (pH = 6.5). (c − e) ESR spectra of scavenging of ·O2–, ·OH, and H2O2 by DMUP. (f) Evaluation of the O2 yield on account of the interplay of DMUP with completely different concentrations of H2O2. (g) Consultant fluorescence microscopy photographs of DMUP on intracellular ROS. Blue: nucleus of cell, Inexperienced: intracellular ROS. (h) Relative fluorescence quantification outcomes of intracellular ROS by DMUP. (i) Photographs of fluorescence microscopy detection intracellular O2. Blue: nucleus of cell, Purple: hypoxia in cells. (j) Outcomes of O2 content material occupancy evaluation. Information are means ± s.d. (n ≥ 3), ns: no important distinction, **p < 0.01, ***p < 0.001, ****p < 0.0001
Validation of DMUP focused micro organism and imaging capabilities
UBI29 − 41 targets bacterial surfaces by electrostatically adsorbing to phosphatidylglycerol and attracting hydrophobic bacterial membranes. Due to this fact, UBI29 − 41 might be particularly focused to the bacterial floor by DMUP by means of floor grafting, which was additionally the premise for correct bacterial imaging (Fig. 4a). SEM was used to check the interplay between DMUP and P. gingivalis, E. coli, and S. aureus after 30 min of incubation at pH 6.5 (Fig. 4b − d). The corresponding photographs revealed that DMUP was in a position to goal and cling to the floor of all three micro organism. The concentrating on efficacy of DMUP was evaluated by loading fluorescein isothiocyanate (FITC, denoted as DMUP-F) into DMUP. DMUP-F was co-cultured with P. gingivalis for five, 10, 20, and 30 min and noticed beneath a fluorescence microscope (Fig. S24a). Mixed with the outcomes of the quantitative fluorescence evaluation (Fig. S24b), it was confirmed that DMUP successfully focused micro organism after co-cultivation for 10 min.
The DMUP (200 µg mL− 1) was evaluated for imaging P. gingivalis, E. coli, and S. aureus. As proven in Fig. 4e, all three micro organism have been blended in equal proportions, grouped in several concentrations, and incubated with DMUP for 10 min. A NIR-II small animal imager was used to detect the fluorescence depth of every group. The bacterial focus positively correlated with the fluorescence depth within the 104−109 CFU mL− 1 bacterial focus group (Fig. 4f). In distinction, the fluorescence depth of the ten1–104 CFU mL− 1 bacterial focus group was too weak to be recorded. Furthermore, there was no important distinction within the fluorescence depth within the 109–1011 CFU mL− 1 bacterial focus group. This lack of distinction was seemingly because of the restricted acidity of the setting across the micro organism, which didn’t lower indefinitely with the elevated bacterial focus [45]. Due to this fact, the findings of the experiments indicated that the efficient bacterial detection focus was 104 CFU mL− 1 or extra when utilizing a DMUP focus of 200 µg mL− 1.
The classical mannequin of bacteria-induced periodontitis in rats was used to judge the impact of DMUP for imaging micro organism in periodontal pockets (Fig. 4g and h). The outcomes confirmed that DMUP nonetheless had the power to picture micro organism within the rugged construction of the periodontal pockets. The Con group had the best fluorescence depth, whereas the DMUP group had the bottom. Then, comparative bacterial plating experiments have been carried out on the periodontal pockets of various teams to judge bacterial load variations. Determine S25 confirmed that the DMUP group exhibited the strongest antibacterial impact, according to the depth of fluorescence within the corresponding group. These outcomes supported DMUP’s concentrating on and imaging capabilities in periodontal an infection websites. Nonetheless, the periodontal pockets of rats have been too small, making it tough to watch bacterial concentration-dependent modifications in fluorescence depth. To raised simulate larger-scale in vivo imaging, we chosen an SD rat leg muscle an infection mannequin. This mannequin reveals robust similarities to periodontal tissue infections, resembling extra ROS, hypoxic situations, and the coexistence of anaerobic and cardio micro organism [46]. The micro organism displayed outstanding proliferation when injected at 106 CFU mL− 1 within the rat (Fig. S26). Moreover, the muscle fibers have been infiltrated with many inflammatory cells (Fig. S27), indicating that injecting 1 mL micro organism (106 CFU mL− 1) or extra may efficiently create a mannequin leg muscle an infection. To additional confirm the efficiency of in vivo bacterial imaging, as illustrated in Fig. 4i, completely different concentrations of blended micro organism have been imaged within the leg muscle after injecting 500 µL DMUP (200 µg mL− 1). The imaging outcomes of small animals, analyzed by the fluorescence depth ratio, confirmed a optimistic correlation with the injected bacterial concentrations, indicating that DMUP may successfully picture the micro organism inside the irritation (Fig. 4j).
Imaging capabilities of DMUP-targeted micro organism. (a) Schematic of DMUP-targeted micro organism and acid-responsive fluorescence imaging. Consultant SEM photographs of DMUP concentrating on (b) P. gingivalis, (c) E. coli, and (d) S. aureus. (e) NIR-II fluorescence photographs of DMUP on completely different concentrations of blended micro organism, and (f) quantification outcomes of fluorescence depth. (g) NIR-II photographs of contaminated areas of periodontitis in rats, and (h) the outcomes of fluorescence relative depth. (i) NIR-II photographs of the bacterial an infection mannequin within the leg, and (j) the outcomes of fluorescence relative depth. Information are means ± s.d. (n ≥ 3), ns: no important distinction, *p < 0.05, **p < 0.01
Examine of antimicrobial and anti-biofilm exercise of DMUP
Micro organism and plaque as initiators of periodontitis. The antimicrobial exercise of DMUP was examined on the next micro organism: P. gingivalis (typical pathogenic anaerobic micro organism), S. aureus (typical cardio micro organism), and E. coli (typical facultative anaerobic micro organism). First, exogenous H2O2 (1 mM) was added to the medium and the pH was adjusted to six.5. A titrimetric methodology was utilized to look at the antimicrobial exercise of various concentrations of DMUP towards 105 CFU mL− 1 of P. gingivalis, E. coli, and S. aureus. The outcomes revealed that the minimal inhibitory concentrations of DMUP towards P. gingivalis and E. coli have been 200 µg mL− 1 and 1600 µg mL− 1, respectively (Fig. S28). In distinction, the antimicrobial effectivity towards the cardio S. aureus was decrease, with no important bacterial inhibition at 1600 µg mL− 1, which was in line with the antimicrobial wants of periodontitis. Because the most important causative organisms of periodontitis are anaerobes, therapy focuses on anti-anaerobes. Due to this fact, at the side of the biosafety focus, we selected the bottom antimicrobial focus of DMUP (200 µg mL− 1) towards the anaerobic bacterium P. gingivalis for subsequent experiments.
SEM was used to watch the completely different levels of cell membrane rupture and contraction of P. gingivalis, E. coli, and S. aureus by 200 µg mL− 1 of DMUP (Fig. 5a). As proven in Fig. 5b, the unfold plate methodology revealed antimicrobial charges of about 99.9%, 95.7%, and 63.5%. This indicated that DMUP exhibited various antimicrobial actions towards the three micro organism. Notably, the inhibition of the anaerobic micro organism by DMUP was superior to that proven by a standard drug and the MC group. Furthermore, since plaque biofilm was thought-about to be the initiating issue of periodontitis, the effectivity of DMUP for eradicating P. gingivalis biofilm was examined by reside/useless staining (Fig. S29 and S30) and crystal violet (CV) staining experiments. As proven in Fig. 5c and d, the outcomes revealed that with a elimination fee of about 96%, DMUP exhibited the perfect elimination effectivity towards P. gingivalis biofilm. Towards E. coli and S. aureus, the elimination charges have been about 81%, and 27%, respectively. Thus, this indicated that DMUP exhibited superior efficiency in eradicating anaerobic bacterial biofilm.
This examine additionally elucidated the antibacterial mechanism of DMUP. The destruction of bacterial cell membranes in a biofilm was investigated with a protein leakage assay. The outcomes revealed that protein leakage was according to these of the anti-biofilm experiments (Fig. S31–S33). Subsequently, the expression modifications in genes associated to P. gingivalis, together with fimbrial protein A (fimA), keratinsulfate glycoprotein (kgp), arginine-specific gingipain A (rgpA), and arginine-specific gingipain B (rgpB), have been detected after therapy. The evaluation revealed that DMUP successfully decreased the expression ranges of the above genes (Fig. 5e–h). Thus, the findings prompt that DMUP destroyed the cell membranes of anaerobic micro organism by inhibiting the expression of the virulent genes of P. gingivalis. With an antibacterial effectivity larger than that of standard drug MC, DMUP exhibited a superb anti-biofilm impact towards anaerobic micro organism. Nonetheless, its antibacterial efficiency towards cardio micro organism was restricted. As periodontitis progresses, anaerobic micro organism primarily contribute to tissue destruction [47]. Thus, the superior efficiency of DMUP in inhibiting anaerobic micro organism was according to the necessities for periodontitis therapy.
Antibacterial and anti-biofilm exercise of DMUP. (a) Consultant plate photographs and SEM photographs after co-incubation of DMUP with P. gingivalis, E. coli, and S. aureus, respectively. (b) Outcomes of antimicrobial properties after quantification. (c) Consultant gentle microscopic photos of CV staining of three biofilms. (d) Anti-biofilm charges of various teams for the three biofilms. (e–h) Adjustments within the expression of P. gingivalis virulence genes after therapy in several teams. Information are means ± s.d. (n ≥ 3), ns: no important distinction, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Experimental research of anti-inflammatory in vitro
A number of signaling pathways, resembling NF-κB and MAPK, are activated by extra ROS generated by an inflammatory response, which promotes the expression of inflammatory components [48, 49]. ROS clearance can block intracellular signaling capabilities and harm mobile constructions, assuaging signs and retarding illness development [50, 51]. Thus, an necessary element of the therapy of inflammatory and immune illnesses includes controlling ROS ranges and lowering the expression of inflammatory components. On this examine, the activated irritation was modeled with the mouse macrophage cell line RAW264.7 cells. The anti-inflammatory capability of DMUP in vitro was decided by inspecting the gene expression of key pro-inflammatory and anti inflammatory mediators within the inflammatory course of after inducing irritation within the RAW264.7 cells (Fig. 6a). Within the quantitative real-time polymerase chain response (qRT-PCR) outcomes, the gene expression of pro-inflammatory components tumor necrosis factor-α (TNF-α), reworking development factor-β (IL-1β), and interleukin-6 (IL-6) elevated considerably in LPS-treated RAW264.7 cells, whereas the gene expression of anti-inflammatory issue reworking development factor-β (TGF-β) decreased comparatively (Fig. 6b and c and S34). After DMUP (200 µg mL− 1) therapy, there was a big lower within the expression of TNF-α, IL-1β, and IL-6. In distinction, expression of the anti-inflammatory issue TGF-β was elevated. Moreover, the expressions of TNF-α and IL-6 have been considerably decreased within the DMU group. In distinction, the expression of IL-1β was not statistically completely different from that of the LPS group. The gene expression of TNF-α and IL-1β was considerably extra decreased within the Pae group than that within the DMU group. Nonetheless, the discount within the expression of IL-6 was decrease within the Pae group than that within the DMU group. The modifications within the expression of the three pro-inflammatory components revealed that the DMUP group displayed the strongest skill to scale back them. Though there have been variations between the Pae and DMU teams within the expression of the pro-inflammatory issue TGF-β, DMUP had probably the most pronounced impact on its elevation. This could be attributed to the synergistic anti-inflammatory impact of Mn nanozyme and polyphenolic antioxidants within the DMUP system.
The impact of DMUP on the immune operate of RAW264.7 cells was examined by performing qRT-PCR assays to detect the M1-type and M2-type markers inducible nitric oxide synthase (iNOS) and arginase 1 (Arg-1) (Fig. 6d and e). Moreover, immunofluorescence (IF) assays have been carried out to detect cluster of differentiation 86 (CD86) and cluster of differentiation 206 (CD206) (Fig. 6f). The outcomes exhibited a rise within the depth of the M1-type macrophage markers iNOS and CD86. In distinction, the depth of the M2-type macrophage markers, Arg-1 and CD206, decreased. This led to a rise within the M1/M2 ratio, which was indicative of a pro-inflammatory profile. The expression of anti-inflammatory components in Fig. 6b–g, displaying that the Pae group (the pure antioxidant) decreased the expression of genes of M1-type inflammatory cells and promoted the manufacturing of M2-type anti-inflammatory cells, in comparison with the LPS group. Mn nanoenzyme mimicked CAT and SOD enzyme actions [24], successfully scavenging extra ROS within the inflammatory setting. This property of Mn nanoenzyme regulated redox stability, decreased M1 macrophage-mediated irritation, and promoted M2 macrophage differentiation, which performed an anti-inflammatory and immunomodulatory function. The DMU group (Mn nanoenzyme loaded system) confirmed decrease expression of TNF-α, iNOS, and CD86 (M1 markers) whereas rising TGF-β, Arg-1, and CD206 (M2 markers). These outcomes indicated that Mn nanozymes additionally contributed to macrophage phenotype switching. The outcomes of the DMUP group have been the mixed impact of Mn nanozyme and Pae. Then, the excessive M1/M2 ratio of LPS group was reversed when the macrophages have been cultured with DMUP (Fig. 6g). The above outcomes prompt that DMUP had anti-inflammatory properties that favored anti-inflammation and tissue restore.
In vitro anti-inflammatory efficacy analysis of DMUP. (a) Schematic illustration of induced irritation RAW264.7 cells, in addition to anti-inflammatory and induced autophagy. Created with BioRender. com. Gene expression of (b) pro-inflammatory issue TNF-α and (c) anti-inflammatory issue TGF-β after group therapy. (d) Expression of Arg-1, a consultant gene of mobile anti-inflammatory phenotype M2, and (e) expression of iNOS, a consultant gene of pro-inflammatory phenotype M1. (f) Consultant fluorescence photographs of various therapy teams. (g) Quantitative fluorescence photographs of M1 (CD86), and M2 (CD206) phenotypes of RAW264.7 cells. Information are means ± s.d. (n ≥ 3), ns: no important distinction, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Molecular mechanisms of anti-inflammation and induction of autophagy in vitro
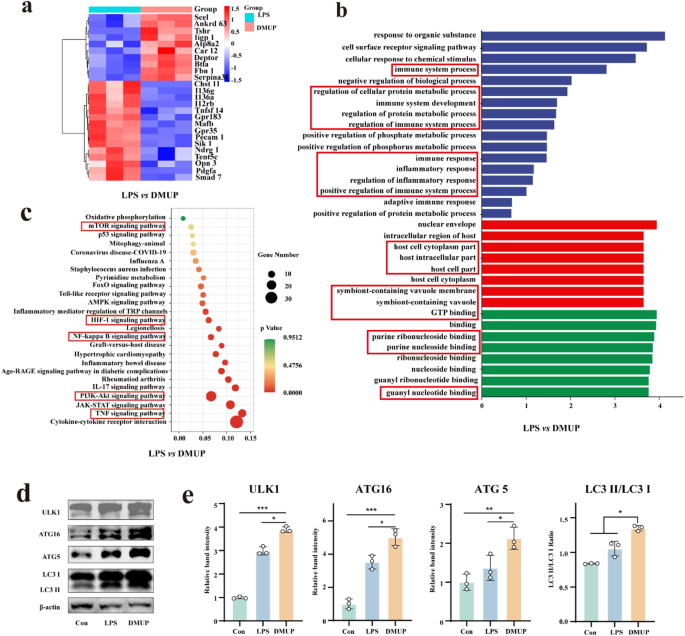
To find out the molecular mechanisms of DMUP anti-inflammatory and autophagy, RAW264.7 cells have been divided into RAW, RAW + LPS, and RAW + LPS + DMUP teams (abbreviated as Con, LPS, and DMUP) for seq-RNA and bioinformatics evaluation induction in vitro. The outcomes of the principal element (PC) evaluation (Fig. S35) confirmed affordable homogeneity inside particular person teams and variations between teams. All three pattern teams met the standard management necessities. Based mostly on the outcomes of the volcano plot (Fig. S36), development plot (Fig. S36), and development warmth map (Fig. 7a and S35), 89 genes have been discovered to be considerably upregulated, and 319 genes have been considerably downregulated within the DMUP group in contrast with the LPS group. Moreover, the outcomes of the gene ontology (GO) evaluation of those differentially expressed genes (DEGs, Fig. 7b) confirmed an enrichment of genes associated to immune, inflammatory, and metabolic responses. The outcomes of the kyoto encyclopedia of genes and genomes (KEGG) evaluation revealed that these genes have been considerably associated to the PI3k-Akt, mTOR, NF-κB, and TNF signaling pathways (Fig. 7c), which additional supported that DMUP performed a job in immune cell autophagy and irritation regulation.
Autophagy is an intracellular degradation course of that reduces inflammatory accidents by way of the elimination of potential pro-inflammatory molecules and maintains intracellular stability in addition to immune homeostasis. The activation of autophagy has the potential to alleviate the expression of inflammatory components, resembling TNF-α and IL-1β. In distinction, autophagy is elevated by the expression of anti-inflammatory cytokines, resembling TGF-β, limiting the over-activation of inflammatory alerts [52]. One of many necessary pathways within the regulation of autophagy is the Akt/mTOR pathway [53], which considerably impacts the transition of macrophages from M1-type to M2-type by way of the regulation of autophagic exercise [54]. Of those, the mechanistic goal of rapamycin advanced 1 (mTORC1) is a serious unfavorable regulator of autophagy, which inhibits autophagy by way of the suppression of key molecules for autophagy initiation (e.g., ULK1). Moreover, mTORC1-mediated inhibition may provoke the autophagy course of, affecting the exercise and purposeful standing of macrophages. This might lead to decreased manufacturing of pro-inflammatory components, suppressing M1-type macrophage pro-inflammatory potential, selling their transition to M2-type macrophages, facilitating the M2-type traits, and enhancing their tissue restore skill and anti inflammatory cytokine manufacturing [55]. Right here, the affect of DMUP on autophagic exercise was confirmed by way of quite a few autophagosomes and autophagic protein markers. The Monodansylcadaverine (MDC) staining outcomes revealed that M1-type macrophages handled by DMUP possessed larger autophagosome content material than untreated M1-type macrophages (Fig. S39 and S40). Subsequent, Western blot (WB) assay was used to find out the expression of autophagic exercise protein markers, autophagy initiation markers (ULK1), autophagic vesicle markers (ATG16, ATG5), and autophagy flux markers (LC3II/LC3I). The relative band intensities prompt that the degrees of ULK1, ATG16, ATG5, and LC3II/LC3I have been elevated in M1-type macrophages handled with DMUP (Fig. 7d and e). This implied that DMUP-activated autophagy and enhanced the autophagic flux in M1-type macrophages. Subsequent, the signaling modifications of Akt and mTOR in DMUP-treated M1-type macrophages have been investigated. Initially, the Akt and mTOR signaling was lively in M1-type macrophages; Nonetheless, after incubation with DMUP, p-Akt and p-mTOR signaling was inactivated (Fig. S41 and S42). The above outcomes prompt that DMUP promoted autophagic exercise by way of the inactivation of the Akt/mTOR signaling pathway to additional forestall macrophage conversion to pro-inflammatory M1-type subtype, facilitating tissue anti-inflammation and restore.
Thus, the multi-enzyme system of DMUP decreased the expression of inflammatory cytokines, enhanced the autophagic flux of immune cells, and controlled each immune cell operate and irritation discount. This, in flip, improved the tissue restore potential and anti inflammatory cytokine manufacturing, preserving the intracellular stability and immune homeostasis and at last lowering the inflammatory harm.
To analyze the molecular mechanisms of DMUP anti-inflammation and autophagy induction on the mobile degree. (a) Clustering heatmap of RNA-seq with gene expression outcomes (LPS vs DMUP), blue: low expression; pink: excessive expression. (b) GO enrichment evaluation (LPS vs DMUP). (c) KEGG enrichment evaluation (LPS vs DMUP), and associated pathways pooled by differential genes. (d) WB assay willpower of autophagy-related protein expression ranges and (e) quantitative evaluation. Information are means ± s.d. (n ≥ 3), *p < 0.05, **p < 0.01, ***p < 0.001
Regulating the impact of the inflammatory setting on in vitro osteogenesis
Throughout irritation, regulation of the mechanisms concerned within the alteration of macrophage polarization standing, autophagy induction, lowering ROS and pro-inflammatory issue ranges, and rising anti-inflammatory issue expression can create a microenvironment favoring osteogenesis [55]. The examine additionally explored the function of DMUP-regulated inflammatory state macrophages within the regulation of osteogenesis in vitro.
As proven in Fig. 8a, the conditioned medium was configured. First, the induction of RAW264.7 cells by LPS to the inflammatory state. Subsequent, DMUP (200 µg mL− 1) was added and cultured for twenty-four h. The supernatant was used to tradition the mouse embryonic osteoblast precursor (MC3T3-E1) cell [56]. The outcomes confirmed that the conditioned medium, post-LPS induction (Con group), decreased the alkaline phosphatase (ALP) exercise of MC3T3-E1 cells. Additionally, the DMUP-treated conditioned medium enhanced ALP exercise of MC3T3-E1 cells (Fig. 8b). The alizarin pink staining (ARS) confirmed related outcomes (Fig. 8c). Their micrographs have been additionally considerably completely different (Fig. 8d). The DMUP-conditioned medium group stimulated the formation of mineralized nodules. Subsequently, the outcomes of the qRT-PCR assay confirmed that DMUP upregulated the expression of osteogenesis-related genes collagen kind I (COL-1), osteocalcin OCN, and runt-related transcription issue 2 (Runx2) (Fig. 8e–g).
The osteopontin (OPN) and Runx2 are two important proteins concerned in osteogenesis, which embody varied osteogenesis-related pathways (wnt/β-catenin, TGF-β/Smad, PI3K/Akt) and affect osteoblast differentiation, bone matrix formation, and mineralization [57]. Right here, a WB assay was carried out to judge the expression of OPN and Runx2. The DMUP-conditioned medium group exhibited elevated expression of OPN and Runx2 in MC3T3-E1 cells (Fig. 8h–j). Thus, the DMUP-conditioned inflammatory microenvironment affected osteogenic metabolism at each the gene and protein ranges, enhancing mineralized nodule formation and selling mobile osteogenic potential.
Impact of DMUP on osteoblast mineralization after adjusting the inflammatory microenvironment. (a) Schematic of conditioned medium preparation. Created with BioRender. (b) Semi-quantitative outcomes of ALP staining. (c) Semi-quantitative outcomes of ARS staining of mineralized nodules, and (d) microscopic staining diagrams. Adjustments within the expression of osteogenesis-related genes (e) COL-1, (f) OCN, and (g) Runx2. (h) WB assay willpower of osteogenesis-related protein expression ranges, and quantification of (i) OPN and (j) Runx2. Information are means ± s.d. (n ≥ 3), ns: no important distinction, *p < 0.05, **p < 0.01, ***p < 0.001
In vivo evaluation of the anti-inflammatory impact of DMUP
The traditional bacterial-induced periodontitis mannequin in rats, utilizing silk-knot and blended bacterial fluids (P. gingivalis, E. coli, and S. aureus) injections, was used to judge the in vivo anti-inflammatory impact of DMUP (Fig. 9a) [55]. Based mostly on the bleeding evaluation by intraoral probing in rats 28 days post-treatment in several teams, the DMUP group exhibited the least quantity of bleeding (Fig. S43). After injecting the bromothymol blue indicator into the periodontal pocket, the infected tissue appeared yellow-green (Fig. S44a and S44b). The pH testing of the supernatant from homogenized contaminated periodontal tissue additionally confirmed a yellow-green shade (Fig. S44c and S44d). In comparison with the colour commonplace of the indicator (Fig. S44e), the pH worth was roughly 6.5, confirming an acidic setting. Blood checks after DMUP therapy confirmed no important modifications in white blood cells (WBC), imply corpuscular hemoglobin (MCH), and platelets (PLT) ranges (Fig. S45). H&E staining of most important organs (Fig. S46) confirmed no irritation or tissue harm within the DMUP-treated group. These outcomes confirmed that DMUP had biosafety in vivo. Subsequently, Masson and hematoxylin-eosin (H&E) staining of periodontal tissues revealed important inflammatory mobile infiltration and collagen loss within the Con group (Fig. 9b and S47), whereas DMUP ameliorated these abnormalities and enhanced collagen deposition. The outcomes of the semi-quantitative analyses additionally confirmed a greater impact than that of the MC group (Fig. 9c and d). Subsequent, immunohistochemical staining was used to find out the expression of pro-inflammatory components (IL-6, IL-1β, and TNF-α) and anti inflammatory components (IL-10) to additional assess the in vivo anti-inflammatory results of DMUP (Fig. 9e and S48–50). The DMUP group confirmed decreased expression of IL-6, IL-1β, and TNF-α and enhanced expression of IL-10, additional confirming that DMUP exhibited a passable anti-inflammatory impact and was stronger than that of the MC group (Fig. 9f and g and S51, S52). The outcomes prompt that DMUP decreased the inflammatory response and promoted periodontal tissue regeneration, thus enjoying an efficient therapeutic function within the bacterial periodontitis mannequin.
With the intention to consider the in vivo osteogenic impact of DMUP, the therapeutic impact of DMUP was evaluated utilizing Micro-CT and histological staining after establishing a classical bacterial-induced periodontitis mannequin in rats. The vertical distance (attachment loss, AL) between the alveolar bone crest (ABC) and the cementoenamel junction (CEJ) was thought-about the therapeutic index by 3D reconstruction [58]. The Con group had the longest distance between ABC and CEJ in contrast with the Con group, displaying important lack of alveolar bone and profitable periodontitis modeling (Fig. S53). DMUP elevated the values of BV/TV, Tb. Th and bone mineral density (BMD) (Fig. 9h–j and S54) and decreased the space between ABC and CEJ in comparison with the Con group. The outcomes of H&E staining of alveolar bone quantity on the heel bifurcation (Fig. S55 and S56) confirmed that DMUP group had the best bone quantity among the many teams. Subsequent, IF was used to check the expression of OCN and Runx2 by completely different teams (Fig. 9ok). The pink fluorescence represented the expression of OCN and Runx2, respectively, and the blue shade represented the DAPI stained cell nuclei. Based mostly on the outcomes of the quantitative fluorescence, the DMUP group exhibited elevated expression of OCN and Runx2, which was considerably larger than that of the MC group (Fig. 9l and m). Thus, DMUP considerably regulated bone formation and bone differentiation-related components, selling bone formation in periodontitis. The MC group additionally exhibited some bone formation results. Nonetheless, these have been considerably completely different from the DMUP group, indicating that the osteogenic impact of DMUP was higher than therapy with MC.
In vivo evaluation of anti-inflammatory osteogenic impact in animals. (a) Schematic diagram of periodontitis modeling on the maxillary left facet of SD rats. Created with BioRender. (b) H&E and Masson staining of maxillary left facet tissues of various teams after therapy, and (c) quantification of the variety of inflammatory cell infiltration and (d) collagen content material of periodontal tissues. (e) Consultant photographs of tissue immunohistochemistry for optimistic expression of IL-6 and IL-10, and (f, g) quantitative outcomes of optimistic expression. (h) Consultant photographs of Micro-CT of maxillary left jaw after therapy in several teams, (i) Tb.Th parameters and (j) BMD values. (ok) Immunofluorescence staining photographs of osteogenesis-related proteins OCN and Runx2 in tissue and (l, m) quantification outcomes. Information are means ± s.d. (n = 6), ns: no important distinction, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001