Preparation and characterization of HM-Lipo-ICG
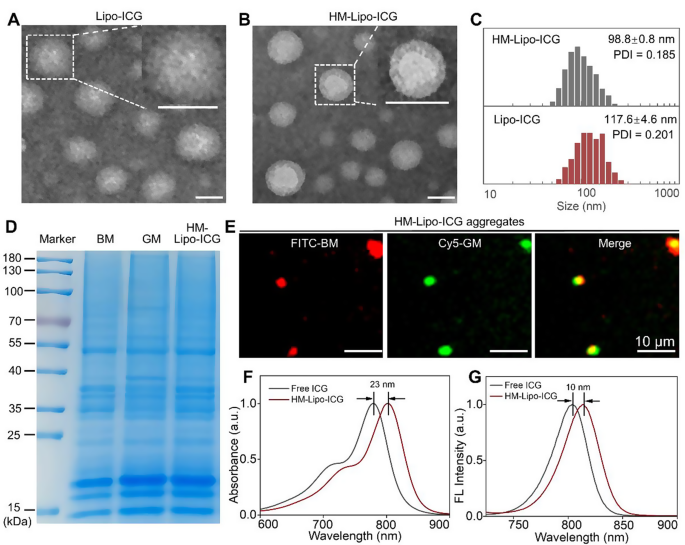
Lipo-ICG was ready utilizing a thin-layer hydration methodology [22, 40]. Cell membranes from B16F10 and G422 GBM cells have been remoted and built-in into Lipo-ICG by steady bodily extrusion, ensuing within the formation of HM-Lipo-ICG [24, 41]. Transmission electron microscopy (TEM) photos revealed uniform spherical shapes with nanoshells of a protein corona in HM-Lipo-ICG, contrasting with Lipo-ICG, confirming profitable membrane integration (Fig. 1A, B). Dynamic gentle scattering (DLS) measurements indicated a smaller hydrated dynamic measurement for HM-Lipo-ICG (98.8 ± 0.8 nm, PDI = 0.145) in comparison with Lipo-ICG (117.6 ± 4.6 nm, PDI = 0.191), attributed to bodily compression throughout membrane modification (Fig. 1C, Fig. S1). HM-Lipo-ICG maintained secure hydrodynamic measurement in water, phosphate-buffered answer (PBS), and 10% fetal bovine serum (FBS) over 10 days, demonstrating glorious stability (Fig. S2). The zeta potential of HM-Lipo-ICG (-36.3 ± 1.0 mV) was larger than that of Lipo-ICG (-45.6 ± 0.4 mV) (Fig. S3), indicating floor potential alteration attributable to membrane incorporation (-37.3 ± 2.9 mV). SDS-polyacrylamide gel electrophoresis (SDS-PAGE) confirmed the presence of major protein bands from B16F10 and G422 cell membranes within the HM-Lipo-ICG band (Fig. 1D), verifying profitable membrane fusion. Moreover, dual-channel fluorescence microscopy of FITC and Cy5 dye-labeled membranes confirmed excessive overlap in HM-Lipo-ICG aggregates (Fig. 1E), confirming profitable co-modification. The encapsulation effectivity and the loading capability of ICG in HM-Lipo-ICG have been calculated to be 77.6 ± 3.7% and three.7% ± 0.2%, respectively.
Characterization of HM-Lipo-ICG. a) TEM photos of Lipo-ICG and b) HM-Lipo-ICG. Samples have been negatively stained with phosphotungstic acid. Insets show a magnified view of a single liposome. Scale bar = 100 nm. c) Hydrodynamic diameter of Lipo-ICG and HM-Lipo-ICG measured by DLS. d) SDS-PAGE protein evaluation of marker, B16F10 cell membrane (BM), G422 cell membrane (GM), and HM-Lipo-ICG. e) Fluorescence microscopy photos of HM-Lipo-ICG aggregates with FITC-labelled B16F10 cell membrane (purple) and Cy5-labelled G422 cell membrane (inexperienced). Scale bar = 10 μm. HM-Lipo-ICG with 50 µL at a focus of 0.1 mg/mL was added dropwise to slides and allowed to dry at room temperature underneath light-protected circumstances. f) Normalized absorption spectra and g) fluorescence spectra of free ICG and HM-Lipo-ICG. CICG = CHM−Lipo−ICG = 20 µg/mL
The photophysical properties of HM-Lipo-ICG have been investigated. UV-vis-NIR absorption and fluorescence spectra have been just like free ICG (Fig. 1F, G), indicating no chemical alteration because of the hybrid membrane coating technique [19]. Nevertheless, an absorption peak redshift from 780 nm to 803 nm (Fig. 1F) and a fluorescence depth redshift from 804 nm to 814 nm (Fig. 1G) was noticed, attributed to free ICG encapsulation within the liposome’s hydrophilic core.
Optical stability evaluation confirmed a big enchancment in HM-Lipo-ICG, with solely a 20% discount in fluorescence depth in comparison with a 65% discount in free ICG after 6 days of storage (Fig. S4), highlighting enhanced stability conferred by liposomal encapsulation.
In Vitro focused BBB penetration of HM-Lipo-ICG
The CD44 protein on the floor of B16F10 cells [42, 43] can particularly work together with the hyaluronic acid (HA) on the floor of bEnd.3 cells [36, 44]. Subsequently, the CD44 marker on the HM-Lipo-ICG was additional detected by western-blotting(WB) measurements. The expression of CD44 in each the BM-Lipo-ICG and HM-Lipo-ICG (Fig. S5) was detected, suggesting that the HM-Lipo-ICG inheritated the marker proteins from supply cells.
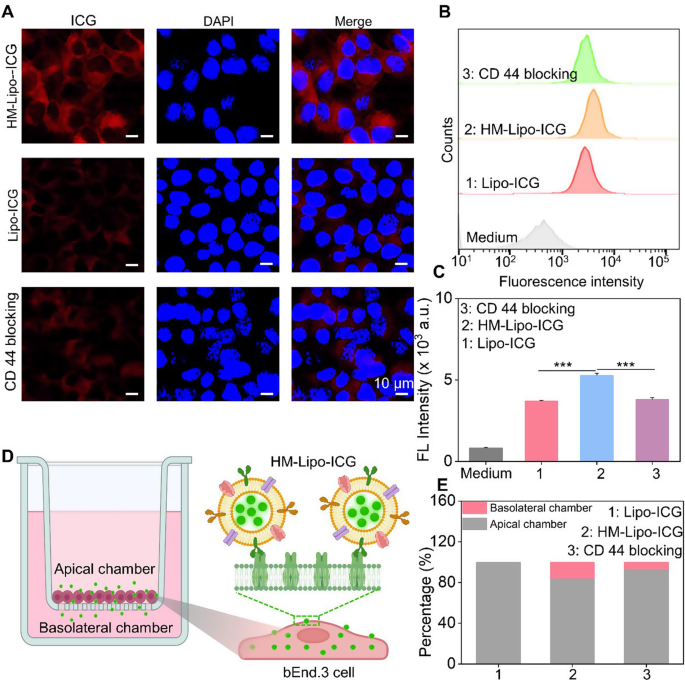
We hypothesized that Lipo-ICG modified with B16F10 cell membranes would improve mobile uptake, thereby selling HM-Lipo-ICG’s means to cross the BBB through receptor-mediated endocytosis [44]. To research this, we first assessed the uptake of HM-Lipo-ICG by bEnd.3 cells utilizing confocal fluorescence imaging and circulation cytometry. Outcomes indicated a better ICG fluorescence sign within the HM-Lipo-ICG-treated group in comparison with the Lipo-ICG-treated group. Conversely, within the CD44 antibody-blocked group, the ICG sign in bEnd.3 cells was considerably decreased in comparison with the HM-Lipo-ICG-treated group (Fig. 2A). Movement cytometry quantitative evaluation revealed that the imply fluorescence depth of the HM-Lipo-ICG-treated group was 1.5 instances larger than that of the Lipo-ICG-treated group and 1.4 instances larger than that of the CD44 antibody-blocked group (Fig. 2B, C). This end result signifies that fusion cell membrane modification enhances the uptake effectivity of Lipo-ICG by bEnd.3 cells, and this enhancement is intently associated to the precise interplay between the HA on bEnd.3 cells and CD44 on B16F10 cell membranes.
In vitro analysis of HM-Lipo-ICG for crossing the BBB. a) CLSM photos of bEnd.3 cells incubated for 4 h with Lipo-ICG, HM-Lipo-ICG NPs, and HM-Lipo-ICG NPs pre-blocked with CD44 antibody. CICG = CLipo−ICG = CHM−Lipo−ICG = 20 µg/mL. The cell nuclei have been stained by DAPI. Scale bar = 10 μm. b) Movement cytometry quantification of bEnd.3 cell uptake of Lipo-ICG, HM-Lipo-ICG, and HM-Lipo-ICG following CD44 antibody blocking. c) Quantitative evaluation of the typical fluorescent depth of ICG in Fig. 2B. Information denote imply ± s.d, ∗∗∗ P < 0.001, n = 3. d) Schematic of the in vitro Transwell mannequin. The inset illustrates the mechanism by which HM-Lipo-ICG crosses the BBB by receptor-mediated endocytosis. e) Quantitative evaluation of the flexibility of Lipo-ICG, HM-Lipo-ICG and HM-Lipo-ICG pre-blocked with CD44 antibody to traverse the in vitro BBB mannequin (n = 3). The quantity of ICG within the higher and decrease chambers was measured and calculated utilizing a linear normal curve
Subsequent, an in vitro BBB Transwell mannequin [44] was established utilizing bEnd.3 cells to preliminarily consider the flexibility of HM-Lipo-ICG to traverse the BBB (Fig. 2D). The trans-endothelial electrical resistance (TEER) of the BBB Transwell mannequin was measured to verify mannequin reliability (knowledge not proven). Outcomes confirmed that the relative proportion of ICG within the apical chamber of the HM-Lipo-ICG-treated group (80.8%) was decrease than that of the Lipo-ICG (100%) and CD44 blocking teams (91.2%) (Fig. 2E). Conversely, the proportion of ICG within the basolateral chamber of the HM-Lipo-ICG-treated group (19.2%) was larger than that of the Lipo-ICG (0%) and CD44 blocking teams (8.8%) (Fig. 2E). This confirms that fusion cell membrane considerably enhances the flexibility of Lipo-ICG to traverse the in vitro BBB.
Homotypic concentrating on of HM-Lipo-ICG to glioma cells in Vitro
Earlier than assessing the homologous concentrating on efficiency of HM-Lipo-ICG, its cytotoxicity to G422 GBM cells and bEnd.3 cells was evaluated utilizing the Cell Counting Package-8 (CCK-8) assay (Fig. S6). The outcomes demonstrated that HM-Lipo-ICG displays excessive cell viability even at an ICG focus of 40 µg/mL, which is eight instances larger than the focus used for in vivo imaging [45]. This discovering underscores the favorable mobile biocompatibility of HM-Lipo-ICG, confirming its suitability for secure use in each tumor imaging and therapeutic functions.
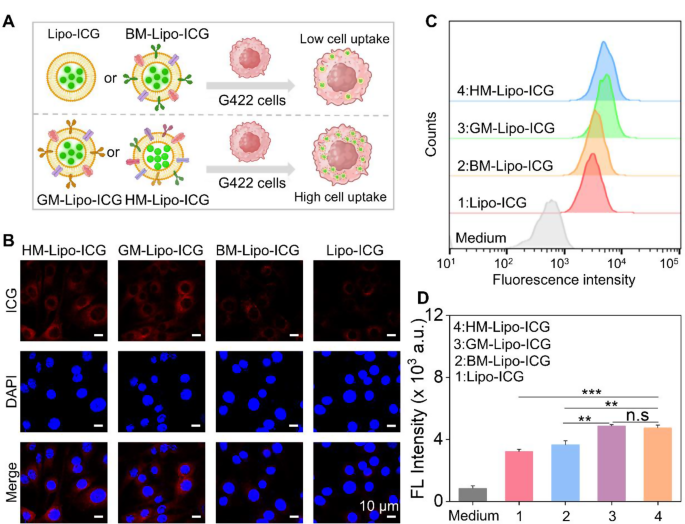
Subsequent, the homologous concentrating on efficacy of HM-Lipo-ICG to G422 GBM cells was investigated utilizing confocal laser scanning microscopy (CLSM) and circulation cytometry. HM-Lipo-ICG, Lipo-ICG, BM-Lipo-ICG, and GM-Lipo-ICG have been incubated with G422 cells for two h, adopted by fixation and DAPI staining (Fig. 3A). The outcomes confirmed a definite fluorescent sign from ICG (proven in purple) within the HM-Lipo-ICG-treated and GM-Lipo-ICG-treated teams, indicating a big enhance in mobile uptake by G422 cells (Fig. 3B). This remark underscores the commendable self-recognition capability of HM-Lipo-ICG and GM-Lipo-ICG in direction of homologous cells, a vital facet for attaining focused most cancers cell capabilities [46, 47]. Quantitative evaluation by circulation cytometry revealed that the typical fluorescence depth in HM-Lipo-ICG and GM-Lipo-ICG-treated cells exceeded that in Lipo-ICG-treated G422 cells by an element of 1.5 (Fig. 3C and D). This additional substantiates that HM-Lipo-ICG and GM-Lipo-ICG, by inheriting cell membrane proteins from G422 cells, effectively enter glioma websites by homologous concentrating on.
Uptake of HM-Lipo-ICG by G422 glioma cells. a) Schematic of G422 glioma cell uptake of Lipo-ICG, BM-Lipo-ICG, GM-Lipo-ICG, and HM-Lipo-ICG. Picture was created with BioRender.com, with permission.b) CLSM photos of G422 glioma cells incubated with Lipo-ICG, BM-Lipo-ICG, GM-Lipo-ICG, and HM-Lipo-ICG for two h. Cell nuclei have been stained with DAPI. CICG = 20 µg/mL. Scale bar = 10 μm. c) Movement cytometry evaluation of G422 cell uptake of Lipo-ICG, BM-Lipo-ICG, GM-Lipo-ICG, and HM-Lipo-ICG. d) Imply fluorescence depth of ICG in G422 glioma cells handled with Lipo-ICG, BM-Lipo-ICG, GM-Lipo-ICG, and HM-Lipo-ICG for two h (n = 3) in Fig. 3C. ns, ∗, ∗∗, and ∗∗∗ symbolize no statistical distinction, P < 0.05, P < 0.01, and P < 0.001, respectively
Moreover, to comprehensively consider the efficiency of HM-Lipo-ICG in a sensible GBM mind situation, an in vitro BBB mannequin was established by seeding endothelial cells within the higher chamber and GBM cells within the decrease chamber (Fig. S7A). Subsequently, HM-Lipo-ICG, GM-Lipo-ICG, and Lipo-ICG have been individually launched into the higher chamber to evaluate their means to penetrate the BBB and be taken up by glioma cells utilizing confocal laser scanning microscopy (CLSM). The outcomes obtained from CLSM imaging (Fig. S7B) and quantitative evaluation (Fig. S7C) demonstrated that the group handled with HM-Lipo-ICG exhibited superior BBB penetration and active-targeting capabilities in comparison with each the GM-Lipo-ICG-treated group and Lipo-ICG-treated group. These findings spotlight that by modification with homologous cell membrane, hybrid cell membrane camouflaged Lipo-ICG demonstrates glorious tumor concentrating on skills after crossing the BBB (Fig. S7).
Lastly, to evaluate whether or not the coating of tumor cell membranes protects towards phagocytosis by macrophages, the mobile uptake of HM-Lipo-ICG by RAW264.7 cells was investigated utilizing CLSM and circulation cytometry. The outcomes revealed that Lipo-ICG-treated cells exhibited elevated purple luminescence, whereas HM-Lipo-ICG-incubated cells confirmed a 1.5-fold discount in intracellular fluorescence (Fig. S8). This implies that solely a minimal variety of cell membrane-coated liposomes are internalized by RAW264.7 macrophages, underscoring the improved phagocytosis-evading capabilities conferred by the biofunctional properties inherited from the supply most cancers cells [46, 48].
In Vivo focused BBB penetration of HM-Lipo-ICG
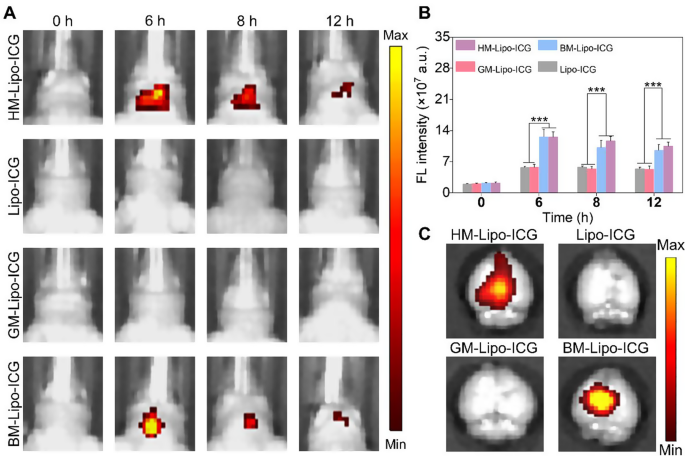
Inspired by the profitable traversal of the in vitro Transwell BBB by HM-Lipo-ICG, we additional evaluated the flexibility of HM-Lipo-ICG to penetrate the BBB and enter mind tissue in a traditional mouse mannequin utilizing NIR fluorescence imaging. 4 completely different liposomal formulations, together with HM-Lipo-ICG, Lipo-ICG, GM-Lipo-ICG, and BM-Lipo-ICG, have been administered through intravenous injection (1.0 mg/kg per mouse), and real-time NIR fluorescence imaging was carried out at varied time factors. As illustrated in Fig. 4A, the teams injected with Lipo-ICG and GM-Lipo-ICG exhibited minimal fluorescence alerts within the mind areas of wholesome mice. In stark distinction, the teams receiving BM-Lipo-ICG and HM-Lipo-ICG confirmed considerably larger fluorescent alerts, confirming that using metastatic most cancers cell membrane camouflage conferred distinctive BBB permeability to the liposomes, permitting them to penetrate the mind even when the BBB was intact. Quantitative evaluation revealed that, at 6 h post-injection, the fluorescence depth within the brains of mice handled with HM-Lipo-ICG and BM-Lipo-ICG was roughly 2.8 instances larger in comparison with these handled with Lipo-ICG and GM-Lipo-ICG (Fig. 4B). No statistically important distinction in fluorescence depth was noticed between the Lipo-ICG and GM-Lipo-ICG teams, nor between the BM-Lipo-ICG and HM-Lipo-ICG teams. These findings counsel that cell membrane-coated biomimetic liposomes derived from mind metastatic most cancers cells retain the useful properties of the unique cell membrane, thus enhancing their BBB penetration capabilities. Moreover, ex vivo imaging of dissected brains corroborated the in vivo outcomes, exhibiting substantial fluorescent alerts within the BM-Lipo-ICG and HM-Lipo-ICG teams (Fig. 4C).
HM-Lipo-ICG crossing the BBB in regular mice. a) NIR fluorescence imaging of regular mouse brains at varied time factors following intravenous injection of HM-Lipo-ICG at a dose of 1.0 mg/kg. b) Quantitative evaluation of fluorescence depth in mouse brains from Fig. 4A. n = 3. c) Ex vivo NIR fluorescence photos of mind tissue from completely different therapy teams 6 h post-intravenous injection
In vivo imaging of infiltrative tumor margins
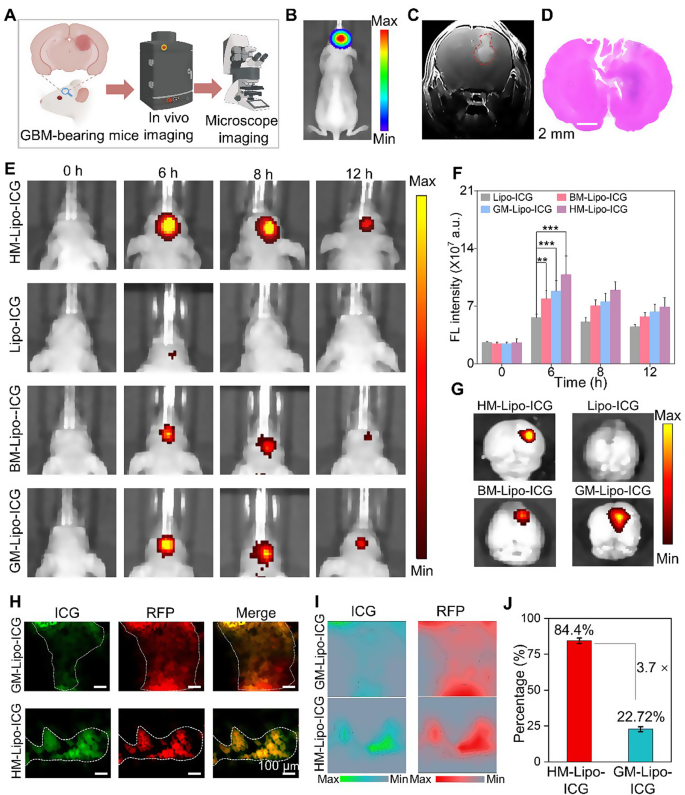
The G422 orthotopic glioma mouse mannequin was established utilizing strategies beforehand described by our group [22]. This mannequin was utilized to judge the in vivo and microscopic near-infrared (NIR) fluorescence imaging capabilities of HM-Lipo-ICG for visualizing tumor margins (Fig. 5A). Luciferase-stably transfected G422 cells exhibited robust bioluminescent alerts within the mouse mind 9 days post-establishment (Fig. 5B and Fig S9). Gadolinium-based T1-weighted contrast-enhanced magnetic resonance imaging additionally delineated the mind tumor area in vivo (Fig. 5C). Moreover, histopathological evaluation of complete mind tissue from mannequin mice utilizing hematoxylin and eosin (H&E) staining revealed areas of infiltrative tumor margins throughout the mind tissue (Fig. 5D). These findings collectively verify the profitable institution of an infiltrative GBM orthotopic mouse mannequin.
HM-Lipo-ICG for correct visualization of infiltrative tumor margins in an orthotopic glioma mouse mannequin. a) Schematic of the G422 orthotopic glioma mannequin development and its utility in fluorescence and microscopic NIR fluorescence imaging in vivo. b) A bioluminescence picture and c) a magnetic resonance picture of a G422 orthotopic glioma mouse. d) H&E-stained histological part of mind tissue of G422 glioma cells in mouse brains 9 days post-implantation. Scale bar = 10 μm. e) NIR fluorescence photos of G422 tumor-bearing mice handled with Lipo-ICG, BM-Lipo-ICG, GM-Lipo-ICG, and HM-Lipo-ICG at varied time factors. Intravenous dose of ICG = 1.0 mg/kg. f) Quantitative evaluation of fluorescence sign depth within the mouse mind areas in Fig. 5E. g) Ex vivo fluorescence photos of mind tissue 4 h post-injection of Lipo-ICG, BM-Lipo-ICG, GM-Lipo-ICG, and HM-Lipo-ICG. h) NIR fluorescence microscopy photos of mind tumor Sect. 6 h post-injection of GM-Lipo-ICG and HM-Lipo-ICG, respectively. Scale bar = 10 μm. i) Quantitative fluorescence depth distribution map of Fig. 5H. The map is obtained by extracting and normalizing the fluorescence depth alerts from Fig. 5D and projecting them onto the corresponding positions. Scale bar = 10 μm. j) Quantitative evaluation of the outcomes from Fig. 5I (n = 3)
Subsequently, the in vivo NIR fluorescence imaging functionality of HM-Lipo-ICG for visualizing infiltrative tumor margins was assessed utilizing the IVIS imaging system. Following intravenous injection of HM-Lipo-ICG (1.0 mg/kg per mouse), NIR fluorescence imaging was carried out at varied time factors (Fig. 5E). The outcomes demonstrated progressive fluorescence alerts throughout the mind tumor area within the HM-Lipo-ICG, BM-Lipo-ICG, and GM-Lipo-ICG therapy teams, peaking at 6 h post-injection earlier than step by step declining. In distinction, minimal fluorescence was noticed within the Lipo-ICG-treated group throughout the mouse mind. Quantitative evaluation indicated that at 6 h post-injection, fluorescence depth on the tumor websites of BM-Lipo-ICG and HM-Lipo-ICG-treated mice was roughly 1.4-fold and 1.9-fold larger, respectively, in comparison with Lipo-ICG-treated mice (Fig. 5F). Following Fig. 4A, the height accumulation of HM-Lipo-ICG within the focused tumor areas is indicated by the very best fluorescence depth noticed at 6 h post-injection. The decline in fluorescence depth over time will be attributed to a mixture of liposome clearance by lymphatic and circulatory techniques, metabolic degradation, and potential mobile uptake. HM-Lipo-ICG enabled high-contrast NIR imaging with a signal-to-background ratio of 6.5 in GBM areas throughout the orthotopic glioma mouse mannequin. Ex vivo imaging of mind tissues from completely different therapy teams corroborated the in vivo fluorescence imaging outcomes (Fig. 5G), confirming the superior homotypic concentrating on means of G422 glioma cell membrane-camouflaged liposomes. Importantly, fluorescence depth in gliomas of the HM-Lipo-ICG-treated group was considerably higher than within the GM-Lipo-ICG and BM-Lipo-ICG-treated teams, underscoring that HM-Lipo-ICG nanoparticles successfully traverse the BBB surrounding rising gliomas and exhibit particular tumor-targeting capabilities.
Moreover, NIR fluorescence microscopic imaging of HM-Lipo-ICG was employed to delineate infiltrative tumor margins in frozen sections of mind tissues. For this goal, RFP-transfected G422 (RFP-G422) cells have been used to ascertain orthotopic glioma mouse fashions. As depicted in Fig. 5H, mind tissue sections from the HM-Lipo-ICG-treated group confirmed full overlap between purple fluorescence emitted by glioma cells and inexperienced fluorescence emitted by ICG. In distinction, inexperienced fluorescence of ICG within the GM-Lipo-ICG-treated group didn’t totally correspond with purple fluorescence at glioma margins, indicating that liposomes modified with hybrid cell membranes can extra precisely determine infiltrative glioma margins in comparison with these modified with single glioma cell membranes. Additional quantitative evaluation (Fig. 5I, J) revealed that the settlement between tumor areas recognized by the HM-Lipo-ICG-treated group and precise tumor areas was as excessive as 84.4%, roughly 3.7 instances larger than that of the GM-Lipo-ICG-treated group (22.7%).
Lastly, the biodistribution and metabolic pathways of HM-Lipo-ICG in regular mice have been evaluated. Ex vivo NIR fluorescence imaging (Fig S10) revealed that 6 h post-intravenous injection, HM-Lipo-ICG primarily collected in liver tissue, in line with findings for Lipo-ICG, BM-Lipo-ICG, and GM-Lipo-ICG. Nevertheless, sign depth in liver tissue was decrease within the HM-Lipo-ICG-treated group in comparison with the Lipo-ICG-treated group, suggesting that cell membrane modification can cut back reticuloendothelial system (RES) uptake of Lipo-ICG, thereby enhancing imaging high quality.
Fluorescence image-guided surgical resection of GMB in vivo
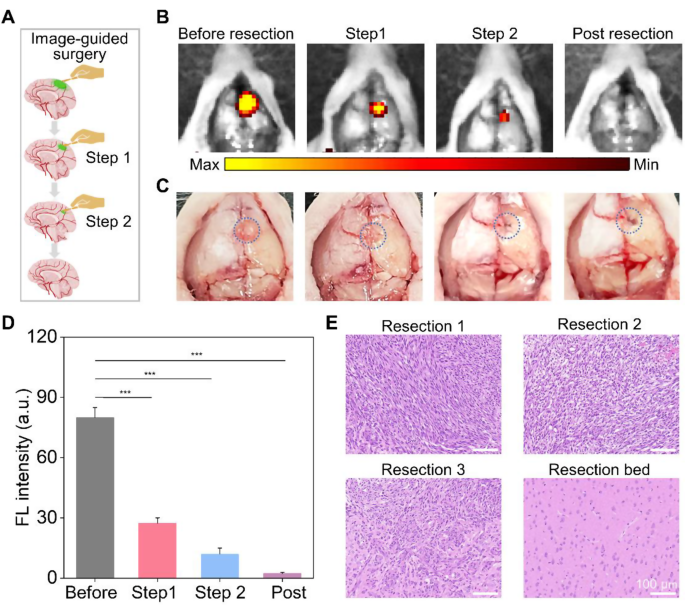
HM-Lipo-ICG demonstrated a outstanding means to determine infiltrative tumor margins, highlighting its potential for guiding fluorescence imaging-directed surgical resection. To evaluate its applicability in facilitating exact neurosurgery for GBM, craniotomies have been carried out with stepwise glioma tissue elimination guided by NIR fluorescence imaging of HM-Lipo-ICG, as depicted in Fig. 6A. A consultant mind bearing GBM was collected 6 h post-injection of HM-Lipo-ICG. The tumor area, highlighted by a blue circle in Fig. 6C, was uncovered and subjected to sequential resections to excise GBM tissue. Earlier than preliminary resection, the tumor protruded from the mind floor, emitting robust fluorescence alerts (Fig. 6B). Following the primary resection, the tumor mass visibly disappeared underneath direct remark, but important fluorescence endured within the resection mattress. Subsequently, a second resection eliminated remaining fluorescent tissue, making a small cavity on the mind floor. Nevertheless, a faint space of residual fluorescence was detected after the second resection, indicating a residual tumor within the surgical cavity. Subsequently, a 3rd resection was carried out, leading to full disappearance of fluorescence. Quantitative evaluation revealed that stepwise glioma elimination decreased fluorescence on the tumor web site from 80.0 (pre-resection) to 27.4 (first resection), 12.0 (second resection), and in the end to zero (Fig. 6D). To validate surgical outcomes, resected tissue was examined utilizing hematoxylin and eosin (H&E) staining (Fig. 6E). Preliminary H&E staining of dissected tumor tissue revealed a dense inhabitants of tumor cells, step by step diminishing with successive resections. Following disappearance of fluorescence, the resection mattress displayed regular mind tissue. These findings emphasize that HM-Lipo-ICG-based NIR fluorescence imaging represents a promising technique for guiding exact and complete neurosurgery, successfully concentrating on gliomas and delineating tumor margins.
In vivo NIR fluorescence imaging-guided glioma surgical procedure in G422 orthotopic glioma fashions. a) Schematic illustration of the stepwise surgical process. b) and c) Digital and NIR fluorescence photos of the tumor resection web site, taken 6 h post-injection of HM-Lipo-ICG nanoparticles. The blue dashed circle in Fig. 6C delineates the tumor location. The surgical procedure continued till all areas emitting a vivid fluorescent sign have been fully excised. d) Quantitative evaluation of fluorescence sign depth within the mouse mind areas in Fig. 6B. e) Consultant H&E-stained sections of the resected tumor tissues following every surgical stage. Scale bar = 100 μm
In vivo toxicity analysis
The in vivo cytotoxicity of Lipo-ICG, GM-Lipo-ICG, BM-Lipo-ICG, and HM-Lipo-ICG was rigorously assessed by a complete evaluation of blood biochemistry. Mice handled with PBS because the management. Outcomes confirmed that the mice handled with nanoparticles exhibited negligible modifications in three key liver operate markers in comparison with the management group (Fig. S11), together with alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP). Moreover, routine blood parameters, together with purple blood cell (RBC) depend, white blood cell (WBC) depend, hematocrit (HCT), imply corpuscular quantity (MCV), hemoglobin (HGB), and platelet depend (PLT), confirmed no important variations between teams. These findings underscore the favorable in vivo biosafety of those nanoparticles, with out inducing distinct liver or renal toxicity. Histopathological examination from H&E-stained slices of main organs (coronary heart, liver, lung, spleen, and kidney) additionally revealed no discernible pathological tissue modifications or injury within the completely different handled teams in contrast with the management group (Fig. S12), indicating the absence of negative effects or toxicities induced by these liposomes or cell membrane coating. Taken collectively, these outcomes verify that HM-Lipo-ICG displays glorious biosafety and that the hybrid tumor cell membrane cloaked technique didn’t have an effect on the conventional operate of the human tissues, holding important promise for medical utility.