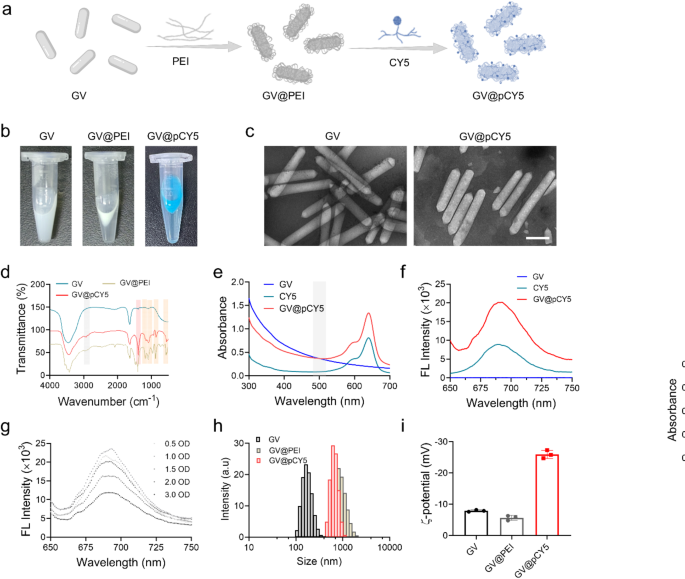
Characterization of GV@pCY5
GVs are rod-shaped, gas-filled protein nanostructures which were utilized in ultrasound-triggered biomedical functions [25]. CY5, a far-red fluorescent dye, is an efficient tracer for peptides and proteins in vivo as a result of its excessive tissue penetration capabilities for bioimaging [26]. Leveraging the distinctive properties of GVs and CY5, we developed a dual-modal imaging probe, GV@pCY5, by means of layer-by-layer (LBL) meeting (Fig. 2a). On this course of, PEI served as each a linker and a protecting agent. GVs extracted from M. aeruginosa have been initially within the type of a milky white suspension in PBS. After PEI grafting by way of electrostatic adsorption, the suspension shortly floated, and turned blue following CY5 meeting (Fig. 2b), indicating the profitable synthesis of GV@pCY5. The GVs and GV@pCY5 have been sometimes rod-shaped, measuring roughly 80 nm in width and 300–800 nm in size. In comparison with the morphology of GV, GV@pCY5, as a result of meeting of PEI and CY5, introduced a number of floor dots (Fig. 2c), but retained the same form to GV, indicated that the synthesis of GV@pCY5 minimally impacts the unique construction and morphology of GV [27].
Characterization of GV@pCY5. a Schematic illustration of self-targeting GV@pCY5 for analysis of glioma. b {Photograph} of GV, GV@PEI, and GV@pCY5 in PBS. c Consultant transmission electron microscopy (TEM) photographs of GV and GV@pCY5. Scale bar: 200 nm. d Fourier rework infrared (FTIR) spectra of GV, GV@PEI, and GV@pCY5. e, f Ultraviolet-visible (UV-vis) (e) and FL (f) spectrum of GV, CY5 and GV@pCY5. Excitation: 630 nm. g FL spectrum of GV@pCY5 at totally different concentrations. Excitation: 630 nm. h, i Dimension distribution (h) and z-potential (i) of GV, GV@PEI and GV@pCY5
Electrostatic interactions between the negatively charged GV and positively charged PEI through the preparation of GV@PEI can affect the molecular exchanges on the protein shell of the GVs, doubtlessly altering their stability. To discover the impact of PEI density on GV stability, we assessed the scale distribution and US imaging efficiency of GV@PEI synthesized with various PEI concentrations. The PEI shell, which scatters gentle upon laser irradiation, resulted in a bigger hydrated dimension for GV@PEI in comparison with naked GVs. The smallest common hydrated dimension and biggest stability have been noticed at a PEI focus of three.75 µg/mL (Determine S1 and Determine S2). Moreover, GV@PEI synthesized with 3.75 µg/mL PEI demonstrated the strongest US imaging distinction (Determine S3 and Determine S4). We supposed that PEI strengthened US imaging by stabilizing the inherent construction of GV by interacting with gasoline vesicle proteins. Nonetheless, the biocompatibility of PEI continues to be difficult the in vivo functions though it was thought-about as a positive macromolecule for biomedical functions [28,29,30]. We additional evaluated the cytotoxicity of GV@PEI (OD500 = 2.0) synthesized from gradient PEI, and located that GV@PEI hardly affected the cell viability of Raw264.7 and GL261 cells even at a excessive PEI focus as much as 7.50 µg/mL (Determine S5). General, PEI at a focus of three.75 µg/mL carried out not solely as a linker between GV and CY5, but in addition improved the US imaging capabilities whereas sustaining low cytotoxicity.
By advantage of the excessive affinity of PEI with lipid-soluble dyes [31], CY5 was linked onto GV@PEI to generate GV@pCY5. To substantiate the linkage between CY5 and GV, we recorded the FTIR spectra of GV, GV@PEI, and GV@pCY5. Peaks at 2935 and 2832 cm-1 appeared within the FTIR spectra of GV@PEI and GV@pCY5 (Fig. 2d), contributing to the stretching vibration of -CH2 in PEI [32]. In addition to, peak at 1190 cm-1 within the spectrum of GV@PEI and GV@pCY5 belonged to the C-N stretching vibration peaks of PEI and CY5. Moreover, peaks at 1468, 950, 721, 636 cm-1 indicated bending vibration of -NH disappeared within the spectrum of GV@pCY5, and peaks at 1350, 1077, 860, and 547 cm-1 indicated the existence of -SO2-, S = O, S-O, and S-C, ascribing to the attribute construction of CY5 [33]. The above outcomes advised that CY5 was linked on GV by way of bodily interplay with out forming new chemical bond. We then verified the existence of CY5 on GV by detecting the UV-vis and FL spectrum of CY5 and GV@pCY5. A consultant absorbing peak of CY5 will be noticed at 630 nm on the UV-vis spectra of GV@pCY5 (Fig. 2e), and the emission peak at 690 nm was detected on the FL spectra of GV@pCY5 (Fig. 2f). In addition to, GV@pCY5 (OD500 = 2.0) confirmed a 2.0-fold FL enhancement in comparison with free CY5, implying that the nanostructured GV may enhance the FL depth of CY5 (Fig. 2f and g). Though GV confirmed the same morphology to GV@pCY5 in TEM photographs, their hydrated diameters have been 182.8 ± 9.2 and 808.4 ± 44.9 nm, respectively (Fig. 2h). The bigger dimension of GV@pCY5 could possibly be defined by the formation of PEI hydration layer on GV. The z-potential of hybrids decreased largely from − 7.50 ± 0.30 to -25.9 ± 1.13 mV following ornament with PEI and CY5 (Fig. 2i). Taken collectively, the stabilized dual-modal FL/US imaging probe was efficiently synthesized by LBL meeting, which possessed increased FL and US imaging skill compared to free CY5 and bared GV.
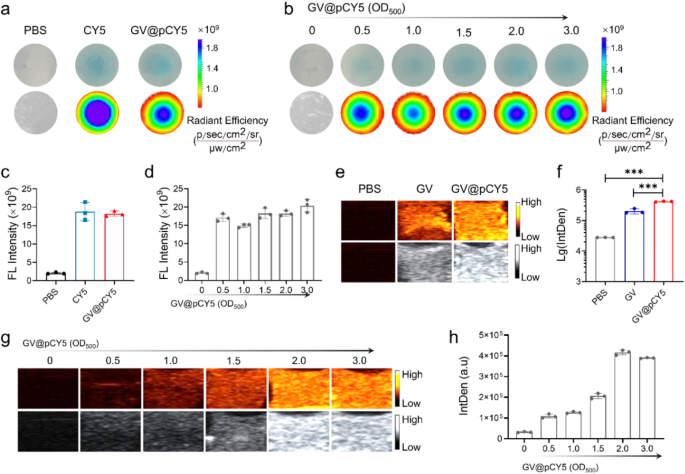
FL and US imaging efficiency of GV@pCY5
To evaluate the in vitro FL/US imaging capability of the dual-modal imaging probe, we recorded the FL and US photographs of GV@pCY5 by dwelling imaging gadgets. PBS, CY5, and GV@pCY5 have been sealed in gels comprised of agarose to simulate tumors earlier than FL and US imaging in response to the literature [24]. When sealed within the in vitro gel fashions, GV@pCY5 possessed a extra even FL imaging capability than free CY5 (Fig. 3a) along with an equal FL depth (Fig. 3c), illustrating that GV@pCY5 may pose a prospect in FL imaging. The imaging skill of FL imaging probes could be influenced by their focus [34], subsequently, we evaluated the corresponding affect of the focus on FL imaging of GV@pCY5. Specifically, the FL imaging skill of GV@pCY5 confirmed a dose-independent habits, as they confirmed comparable FL photographs (Fig. 3b) and equal FL depth (Fig. 3d).
FL/US imaging capability in vitro. a, c FL imaging (a) and FL depth (c) of PBS, CY5, and GV@pCY5 sealed in agarose gels. b, d FL imaging (b) and FL depth (d) of GV@pCY5 sealed in agarose gels in 0, 0.5, 1.0, 1.5, 2.0, and three.0 OD500. e, f US imaging (e) and US IntDen (b) of PBS, CY5, and GV@pCY5 sealed in agarose gels. g, h US imaging (g) and US IntDen (h) of GV@pCY5 sealed in agarose gels in 0, 0.5, 1.0, 1.5, 2.0, and three.0 OD500. n = 3, ***, p ≤ 0.001
We investigated the acceptable output energy of Mindray transportable ultrasound machines by making use of sequence of output energy on agar fashions [35]. The IntDen of each GV and GV@pCY5 elevated together with the enlarged output energy earlier than a 55%-output energy, after which decreased steadily (Determine S6). Consequently, an output energy of 55% was chosen to evaluate the US distinction effectivity each in vitro and vivo. GV and GV@pCY5 confirmed brightest US alerts (Fig. 3g and Determine S7) and highest IntDen (Fig. 3h and Determine S8) at a focus of two.0 OD500. Consequently, we utilized GV@pCY5 at a focus of two.0 OD500 to conduct the mobile and in vivo imaging experiment with an output energy of 55%. Remarkably, GV@pCY5 owned an improved US imaging skill in contrast with bared GV (Fig. 3e and f), as a result of invigorating impact of PEI on protein skeleton, which was additional confirmed by the centered ultrasonic remedy. Upon US remedy, GV@pCY5 ready from diluted PEI saved their milk-white shade; whereas GVs turned clear (Determine S9), contributing to the injury of the vesicle construction, which indicated that GV@pCY5 possessed a greater anti-ultrasonic stripping property in contrast with bared GV. Collectively, GV@pCY5 confirmed enhanced FL/US imaging capability with increased homogeneity in contrast with bared GV and free CY5 in imaging gadgets, indicating the potential software in enhanced dual-modal imaging.
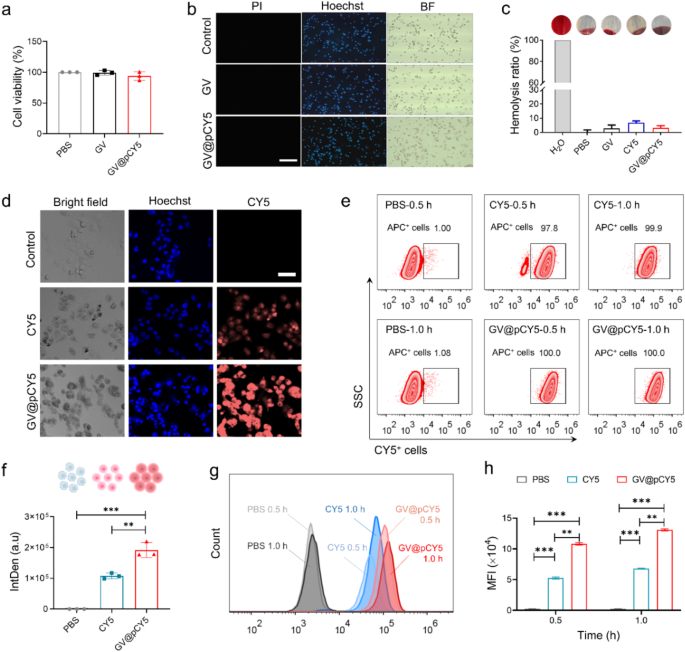
Cytotoxicity, cell uptake and penetrability of GV@pCY5
The imaging capability of probes was associated to their cell viability and environment friendly mobile uptake [36, 37], thus we measured the cytotoxicity, mobile uptake, in addition to hemolysis properties of GV@pCY5 to evaluated its applicability. GV@pCY5 at a focus of two OD500 confirmed no apparent affect on cell viability of Raw267.4 and GL261 cells publish a 24-h-treatment (Determine S10 and Fig. 4a), indicating its biocompatibility on the mobile stage. Reside-dead staining of Raw267.4 cells handled by GV@pCY5 confirmed the identical outcome. We handled 1 × 105 of Raw267.4 and GL261 cells with GV and GV@pCY5 at a focus of two.0 OD500 for twenty-four h, after which stained the cells with combination of pyridine iodide (PI) and Hoechst 33,342 (Hoechst). Beneath FL microscopy, dwelling and lifeless cells appeared as blue and purple shade. The Raw267.4 cells nonetheless alive after they have been handled by GV and GV@pCY5 (Fig. 4b), demonstrating the ultralow cytotoxicity of GV@pCY5 towards Raw267.4 and GL261 cells. To evaluate the sensible chance, we detected the hemolytic price of GV, CY5, and GV@pCY5 by incubating them with purple blood cells. PBS and water have been used as unfavourable and constructive controls. Each GV and GV@pCY5 hardly triggered hemolysis as their hemolytic price was lower than 5%; CY5 would trigger an roughly 7% hemolytic price (Fig. 4c). The outcomes proved that GV mounted CY5 confirmed a decreased hemolytic price in contrast with free CY5, illustrating the next biocompatibility of GV@pCY5 [38]. Consequently, GV@pCY5 have been biocompatible to Raw264.7 and GL261 and purple blood cells when their focus was 2.0 OD500, which might profit the additional in vivo functions.
Inadequate mobile uptake by goal cells has hindered the medical software of nanostructured imaging probes [39]. To deal with this, we evaluated the mobile uptake of CY5 and GV@pCY5 in GL261 and Raw264.7 cells. In comparison with GL261 cells handled with free CY5, these handled with GV@pCY5 exhibited considerably increased fluorescence depth (Fig. 4d). Particularly, a considerable enhance in fluorescence was noticed in GL261 cells handled with GV@pCY5 in comparison with free CY5 (Fig. 4f), indicating a choice of GL261 cells for nano-sized biohybrids over free dyes. In distinction, Raw264.7 macrophages, L929 fibroblasts, and CT26 colorectal most cancers cells confirmed comparable mobile uptake efficacy for each GV@pCY5 and free CY5, as evidenced by comparable common fluorescence intensities in these cells (Determine S11, S12, and S13). Moreover, circulation cytometry evaluation revealed that GVs facilitate the entry of CY5, with CY5+ cells handled with GV@pCY5 demonstrating 100% mobile uptake inside 0.5 h (Fig. 4e). Furthermore, the MFI of GL261 cells handled with GV@pCY5 was 2.2-fold increased than that of cells handled with free CY5 (Fig. 4g and h), additional confirming the improved mobile uptake by GL261 cells. General, GV enhances the mobile uptake of CY5 and exhibits good cell and blood compatibility, underscoring its potential significance for bioimaging [40].
Biocompatibility and mobile uptake and penetrability of GV@pCY5. a Cell viability of GL261 cells handled by PBS, GV, and GV@pCY5 for twenty-four h. b Reside/lifeless staining of Raw264.7 cells handled by PBS, GV, and GV@pCY5. Scale bar: 100 μm. c Hemolysis ratio of PBS, GV, CY5, and GV@pCY5. d, f Confocal photographs (d) and Intden (f) of GL261 cells handled with PBS, CY5, and GV@pCY5. Scale bar: 50 μm. e Move cytometry assay of GL261 cells handled by PBS, CY5, and GV@pCY5 for 0.5 and 1.0 h. g, h Histogram (g) and imply FL depth (MFI) (h) of CY5+ GL261 cells handled by PBS CY5, and GV@pCY5 for 0.5 and 1.0 h. n = 3, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001
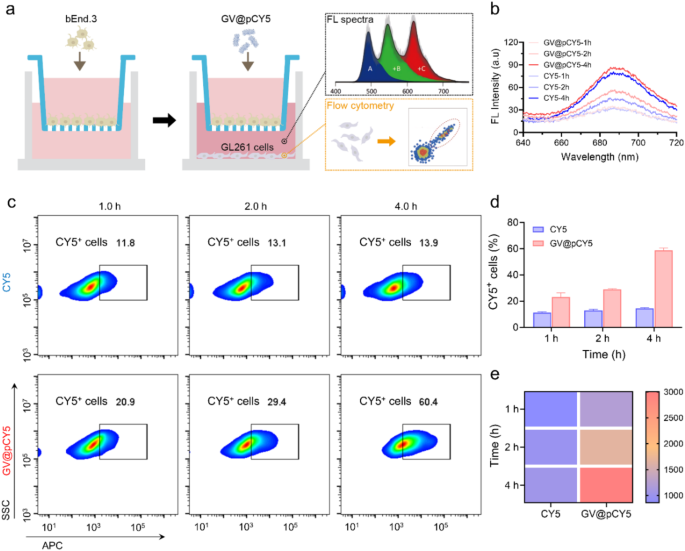
The blood-brain barrier limits the penetration of imaging probes, limiting their imaging efficacy [41]. To evaluate the flexibility of GV@pCY5 to cross this barrier, we established an in vitro mannequin by seeding bEnd.3 cells within the inserts of a transwell plate and culturing them for 10 days. We then added GV@pCY5 to the inserts and picked up the basal medium and cells at varied intervals for fluorescence spectra, dynamic gentle scattering (DLS) evaluation, and circulation cytometry (Fig. 5a). In comparison with free CY5, the basal medium handled with GV@pCY5 confirmed increased fluorescence at sure time factors (Fig. 5b), indicating that GV@pCY5 has improved efficacy in penetrating the blood-brain barrier. In addition to, the presence of PEI contributed to the next particle rely within the basal medium (Determine S14). Moreover, mobile uptake of GV@pCY5 in GL261 cells elevated with incubation time, with roughly 60% of cells exhibiting fluorescence after 4 h of incubation with GV@pCY5 (Fig. 5c and d), which is 40% increased than that noticed with free CY5. The best fluorescence was detected in GL261 cells handled with GV@pCY5 for 4 h (Fig. 5e). General, GV@pCY5 demonstrated superior penetration skill throughout the in vitro blood-brain barrier mannequin in comparison with the free hydrophobic dye and confirmed elevated uptake by glioma cells.
Penetration skill of GV@pCY5 throughout the in vitro blood-brain barrier. a Experimental Design. bEnd.3 cells have been cultured in inserts inside a transwell plate for 10 days till tight junctions have been established. The inserts have been then transferred into wells containing GL261 cells. Following incubation with PBS, CY5, and GV@pCY5, the basal medium and GL261 cells have been collected at specified time factors for additional evaluation. b Fluorescence spectra of the basal medium after incubation with CY5 and GV@pCY5 for 1, 2, and 4 h. c Move cytometry evaluation of GL261 cells following incubation with CY5 and GV@pCY5 for 1, 2, and 4 h. d, e The ratio of CY5+ cells (d) and fluorescence depth (e) in GL261 cells after incubation with CY5 and GV@pCY5 for 1, 2, and 4 h
FL/US imaging in subcutaneous GL261 tumor mannequin
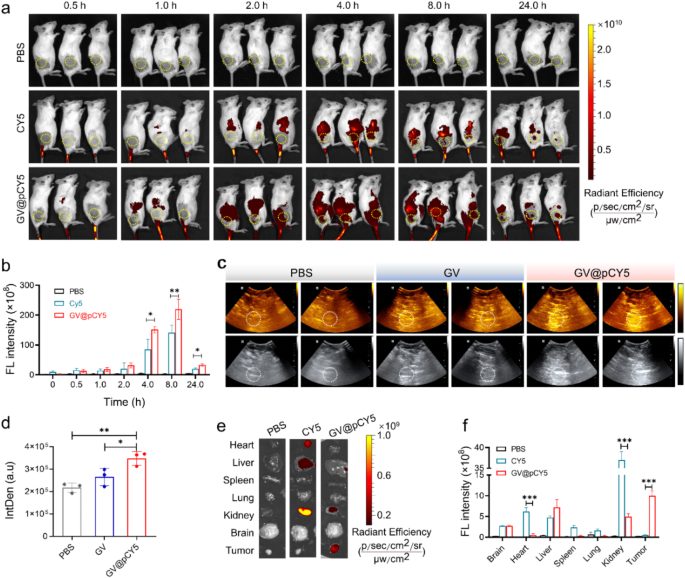
We evaluated the steadiness of GV@pCY5 in FBS over 10 days by monitoring its common dimension and ζ-potential. GV@pCY5 maintained a mean dimension of roughly 800 nm and a ζ-potential of about − 25.5 mV all through the storage interval (Determine S15), indicating good stability and suitability for in vivo functions. To preliminarily examine the US/FL imaging efficiency of GV@pCY5, we handled the subcutaneous GL261 tumor-bearing mice by intravenous administration of PBS, CY5, and GV@pCY5 (100 µL, 2.0 OD500). We recorded the FL photographs by a small animal dwelling imaging system of PerkinElmer in vivo imaging system (IVIS) Lumina III at 0.5, 1.0, 2.0, 4.0, 8.0, and 24.0 h publish administration, and the typical FL sign depth in tumor website was measured by Residing Picture 4.0. The GL261 tumor-bearing mice confirmed sturdy fluorescence at 2.0 h publish injection (Fig. 6a), contributing to the liver accumulation skill of probes [42]. The FL sign at tumor website reached highest stage at 8.0 h and will nonetheless be noticed even at 24.0 h post-injection (Fig. 6b). GV@pCY5 demonstrated increased fluorescence depth at 4 h in comparison with free CY5, suggesting enhanced imaging effectivity. For ultrasound imaging, we used a Mindray DP-50 Skilled transportable ultrasound gadget at 8 h post-injection of PBS, GV, and GV@pCY5. GV@pCY5 produced brighter ultrasound alerts on the tumor website, with better common depth in comparison with GV (Fig. 6c and d), indicating its superior ultrasound imaging functionality. Typically, GV@pCY5 confirmed favorable capability of dual-modal FL and US imaging efficiency, making it a useful device for tumor analysis.
We evaluated the distribution of GV@pCY5 by FL imaging of collected organs and tumors excised from the handled GL261 tumor-bearing mice at 24 h publish injection. A shiny FL sign was noticed in livers and kidneys (Fig. 6e), indicating that the probes have been cleared by means of hepatic and renal metabolism [43]. Remarkably, the typical FL depth on the tumor website of mice handled with GV@pCY5 was 3.4 instances increased than that of the CY5 injected mice (Fig. 6f), illustrating wonderful selective accumulation in tumors of GV@pCY5. These outcomes demonstrated nice potential of GV@pCY5 as an enhanced FL/US dual-modal imaging probe for tumor analysis.
GV@pCY5 enabled improved FL/US twin mannequin imaging of subcutaneous tumors. a FL photographs of mice intravenously injected with PBS, CY5, and GV@pCY5 at totally different time intervals. b Fluorescence depth of the tumor website at 0, 0,5, 1.0, 2.0, 4.0, 8.0, and 24.0 h publish injection with PBS, CY5, and GV@pCY5. c, d Consultant US photographs (c) and IntDen (d) of subcutaneous tumors of mice at 6 h publish intravenous injection with PBS, GV, and GV@pCY5. e, f Fluorescence photographs (e) and fluorescence depth (f) of important organs in mice sacrificed at 24 h publish injection with PBS, CY5, and GV@pCY5. n = 3, *, p ≤ 0.05, **, p ≤ 0.01
Enhanced FL/US imaging in orthotopic GL261 glioma mannequin
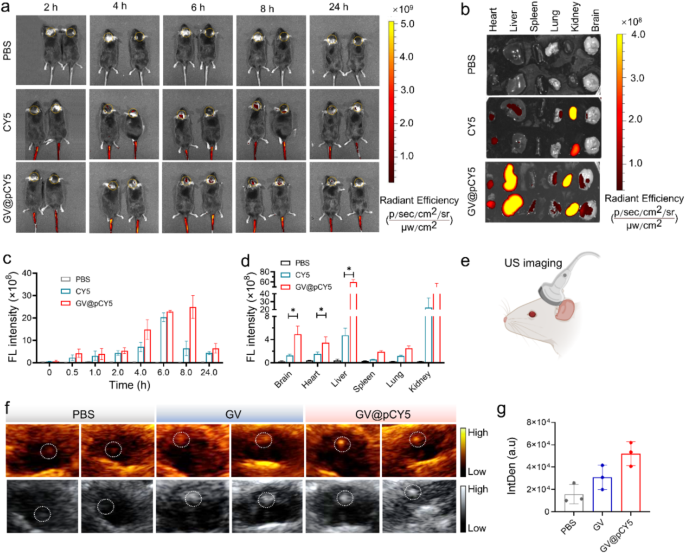
The FL/US imaging skill has been proved each in vitro and in subcutaneous GL261 tumor-bearing fashions, we additional evaluated the FL/US dual-mode imaging skill in orthotopic GL261 glioma fashions. Equally, we recorded each FL and US photographs of orthotopic glioma fashions injected with GV@pCY5. Apparent FL alerts within the tumor website appeared at 2 h publish injection of CY5 and GV@pCY5, and confirmed highest FL depth at 6 h publish injection (Fig. 7a and c). Notably, the tumor website of mice handled with GV@pCY5 possessed 3.0 instances increased FL depth in contrast with mice handled with CY5 at 8 h publish injection (Fig. 7c), illustrating that GV@pCY5 displayed longer analysis window than free CY5 [44]. In addition to, in contrast with free CY5, GV@pCY5 confirmed increased obvious FL alerts on the tumor website of glioma fashions (Fig. 7b and d), indicating the FL imaging effectiveness of GV@pCY5. For US imaging, the mice have been scanned with a Mindray DP-50 Skilled transportable ultrasound imaging gadget (Fig. 7e). The glioma displayed brightest US alerts and clearest tumor boundary amongst mice handled by PBS, GV, and GV@pCY5 (Fig. 7f). Be totally different from FL imaging, US imaging of mice supplied spatialization of glioma, which may strengthen mounted place analysis of intracranial tumors. At 6 h publish intravenous administration, the IntDen of the tumor website of mice injected with GV@pCY5 was 2.2-fold increased than that of mice injected with GV (Fig. 7g), contributing to the excessive stability and US imaging capability of GV@pCY5. The above outcomes indicating that GV@pCY5 confirmed increased effectivity in glioma analysis.
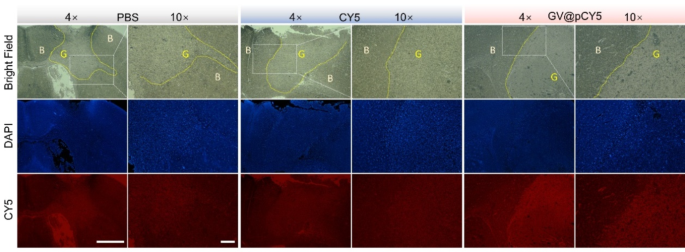
It was suspected that GV@pCY5 confirmed increased accuracy in contrast with free CY5, so we noticed the fluorescence distribution in mind of glioma-bearing mice handled with PBS, CY5, and GV@pCY5. Increased purple fluorescence was detected in tumors of glioma-bearing mice handled with GV@pCY5 compared to free CY5 and PBS (Fig. 8). Free CY5 hardly heightened the FL distinction between mind and glioma, whereas clear boundary appeared in tumors of glioma-bearing mice handled with GV@pCY5, ascribing to the penetrating skill of PEI-capped nano-sized hybrids. Furthermore, excessive distinction between mind and glioma additional verified the self-targeting skill of GV@pCY5. Taken collectively, the simultaneous dual-modal FL and MR imaging may present extra complementary data from every imaging modality for tumor analysis.
GV@pCY5 enabled improved FL/US twin mannequin imaging of orthotopic glioma. a Fluorescence photographs of mice intravenously injected with PBS, CY5, and GV@pCY5 at totally different time intervals. b, d Fluorescence photographs (b) and fluorescence depth (d) of important organs in mice sacrificed at 24 h publish injection with PBS, CY5, and GV@pCY5. c Fluorescence depth of the tumor website at 0, 0,5, 1.0, 2.0, 4.0, 8.0, and 24.0 h publish injection with PBS, CY5, and GV@pCY5. e Diagram of US imaging of orthotopic tumors. f, g Consultant US photographs (f) and IntDen (g) of orthotopic tumors of mice at 6 h publish intravenous injection with PBS, GV, and GV@pCY5. n = 3, *, p ≤ 0.05, **, p ≤ 0.01
Biosafty of GV@pCY5
We additional assessed the in vivo biosafety of GV@pCY5 by inspecting blood, serum, and tissue samples from mice that have been handled with systemic administration of PBS, GV, and GV@pCY5. Evaluation of blood samples from mice administered GV@pCY5 confirmed no vital adjustments within the full blood rely; the degrees of white blood cells (WBC), purple blood cells (RBC), platelets (PLT), and lymphocytes (Lymph) remained steady (Determine S16). HE staining evaluation indicated that GV@pCY5 didn’t trigger notable pathological injury to the liver, spleen, or kidneys in comparison with the management group (Determine S17a). Moreover, biochemical markers associated to liver and kidney perform, akin to ALT, AST, BUN, and UA, have been inside regular ranges for mice handled with GV@pCY5, much like these within the PBS group (Determine S17b-d). Elevated ranges of those markers are sometimes related to liver and kidney injury, additional supporting the low toxicity and favorable in vivo security profile of GV@pCY5 [45]. These findings recommend that GV@pCY5 is a secure and efficient device for in vivo glioma analysis.