Preparation and characterization of MNM/siRNA NPs
Neutrophils, as essentially the most ample leukocyte within the peripheral blood, are the primary cells to be recruited to ischemic harm websites [2, 3]. The mouse neutrophil cell (MNHC) line was chosen for cell membrane coating as a result of its fast recruitment to ischemic websites and interplay with infected endothelial cells through elevated integrins and chemokine receptors [17, 18]. Given the HA’s position in endosomal escape and integrins’ position in recruitment, we utilized adenovirus transfection to precise HA and stimulated integrin α9/β1 expression on neutrophils utilizing ET-1 (Fig. 2(a)). Move cytometry evaluation and immunofluorescence confirmed the expression of HA protein on neutrophils after adenovirus transfection by detecting HA-tagged inexperienced fluorescent protein (HA-GFP, Fig. 2(b-d)). Additional ET-1 stimulation resulted within the overexpression of integrin α9/β1 on neutrophil cells, as detected by western blot (WB) and quantitative polymerase chain response (qPCR) (Fig. 2(e-i)). These outcomes indicated that the neutrophils efficiently acquired ample HA and integrin membrane proteins after the therapy.
Overexpression of HA and integrin α9/β1 on neutrophil cells after adenovirus transfection and ET-1 stimulation. (a) Scheme illustrating the preparation of engineered neutrophil membranes. (b) Move cytometric evaluation of MNHCs co-incubated with adenovirus encoding for HA for 48 h. (c) Move cytometric evaluation of HA expression on MNHCs. MNHC (blue), MNHC + HA (pink). (d) Photographs of MNHCs after transfection with adenovirus encoding for HA for 48 h. HA (inexperienced); scale bar = 50 μm. (e) Consultant immune blots of integrin α9/β1 on MNHCs after ET-1 stimulation for twenty-four h. (f) Quantification of the integrin α9 protein on MNHCs after 24 h of ET-1 stimulation (imply ± SD, n = 5). (g) Quantification of the integrin β1 protein on MNHCs after 24 h of ET-1 stimulation (imply ± SD, n = 5). (h) The mRNA ranges of integrin α9 in MNHCs after 24 h of ET-1 stimulation by qPCR (imply ± SD, n = 5). (i) The mRNA ranges of integrin β1 in MNHCs after 24 h of ET-1 stimulation by qPCR (imply ± SD, n = 5). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (one-way ANOVA with Dunnett’s a number of comparisons take a look at)
Subsequently, we synthesized pABOLs with a molecular weight of 8.1 kDa, chosen for his or her excessive transfection effectivity and low cytotoxicity based mostly on earlier research [8, 19]. Moreover, the pABOLs had been simpler to arrange, lowering each processing time and complexity. These benefits made pABOLs a extra sensible and sturdy platform for siRNA supply, providing an modern and scalable answer for potential therapeutic functions. The siRNA NPs had been then obtained by mixing pABOLs with siRNA. To arrange MNM/siRNA NPs, we remoted the engineered neutrophil membranes utilizing a hypotonic lysis approach, adopted by assembling them with the siRNA NPs by sonication (Fig. 3(a)). To validate the encapsulation of cell membranes on NPs, dynamic gentle scattering (DLS) measurements confirmed that the diameter of the formulated MNM/siRNA NPs was 30 nm better than that of the siRNA NPs, indicating the addition of an additional bilayered cell membrane onto pABOLs (Fig. 3(b)). Earlier research have demonstrated that NPs with diameter sizes between 10 and 200 nm exhibit optimum blood circulation lifespans, with decreased uptake by non-phagocytic cells because the diameter will increase [20, 21]. The floor zeta potential measurements revealed values of -21.63 ± 1.04 mV for MNM, 3.69 ± 0.96 mV for siRNA NPs, and − 6.47 ± 2.46 mV for MNM/siRNA NPs, suggesting the presence of a biomimetic floor cost (Fig. 3(c)). Moreover, DLS measurements confirmed that the formulated MNM/siRNA NPs maintained their dimension over 96 h in phosphate-buffered saline (PBS) and 10% fetal bovine serum (FBS) (Fig. 3(d)), highlighting the colloidal stability of MNM/siRNA NPs in organic situations. Additional confocal laser scanning microscopy (CLSM) photographs confirmed the co-localization of Dil-labeled MNM and Cy5-labeled pABOLs, offering visible affirmation of profitable MNM encapsulation on pABOLs after extrusion (Fig. 3(e)). Transmission electron microscopy (TEM) imaging additionally revealed a cell membrane layer coating the siRNA NPs, which was absent in siRNA NPs alone (Fig. 3(f)). These outcomes collectively indicated the profitable membrane coating strategy of MNM onto pABOLs.
We subsequent evaluated whether or not MNM/siRNA NPs retained key membrane proteins from MNM. Coomassie blue staining revealed that the whole protein composition of MNM/siRNA NPs carefully resembled that of MNM vesicles, confirming the profitable switch of most proteins to the floor of MNM/siRNA NPs through the coating course of (Fig. 3(g)). WB evaluation offered additional validation by detecting the presence of key proteins on MNM/siRNA NPs derived from MNM vesicles, together with integrin α9, integrin β1, lymphocyte function-associated antigen-1 (LFA-1), P-selectin glycoprotein ligand-1 (PSGL-1), HA, C-X-C chemokine receptor kind 2 (CXCR2), and C-C chemokine receptor kind 2 (CCR2), whereas no protein bands had been noticed within the uncoated siRNA NPs (Fig. 3(h)). These findings instructed that MNM efficiently retained the crucial physiological properties of neutrophils.
Characterization of formulated MNM/siRNA NPs. (a) Scheme illustrating the preparation of MNM/siRNA NPs. (b,c) Particle dimension and zeta potential of the MNM, siRNA NPs, and MNM/siRNA NPs (n = 3). (d) Colloidal stability of MNM/siRNA NPs over a span of 96 h in PBS or FBS (n = 3). (e) CLSM photographs of MNM/siRNA NPs with Dil labeled MNM and Cy5 labeled NPs (Scale bar = 20 μm). (f) TEM photographs of MNM/siRNA NPs and siRNA NPs (Scale bar = 200 nm). (g) The whole protein composition of MNM/siRNA NPs confirmed by Coomassie blue staining. (h) WB assay of the important thing proteins on MNM together with integrin α9, integrin β1, LFA-1, PSGL-1, HA, CXCR2, and CCR2
Immune escape and concentrating on means of MNM/siRNA NPs in vitro
Cytotoxicity testing is crucial for evaluating the protection of prescription drugs. We assessed the cytotoxicity of siRNA NPs and MNM/siRNA NPs utilizing the Cell Counting Equipment-8 (CCK-8) technique previous to mobile experiments. The outcomes (Fig. 4(a) and Determine S1(a, b)) demonstrated that concentrations as much as 200 µg/ml of each siRNA NPs and MNM/siRNA NPs had been protected for MNHCs, human umbilical vein endothelial cells (HUVECs), and clean muscle cells (SMCs) after incubation for twenty-four h.
To judge immune evasion, we incubated free siRNA, siRNA NPs, NM/siRNA NPs, and MNM/siRNA NPs with RAW 264.7 cells for 4 h, utilizing Cy5-labeled siRNA. CLSM and circulation cytometry analyses revealed that NM/siRNA NPs and MNM/siRNA NPs considerably lowered macrophage phagocytosis in comparison with free siRNA and siRNA NPs (Fig. 4(b, c), and Determine S1(c, d)), demonstrating the effectiveness of cell membranes in immune evasion.
We then assessed the endosomal escape and degradation avoidance of MNM/siRNA NPs in vitro. MNHCs had been handled with NM/siRNA NPs and MNM/siRNA NPs for various durations following lipopolysaccharide (LPS) administration to simulate activated neutrophils. Cy5, Hoechst 33,342, and LysoTracker Crimson had been used to stain siRNA, nuclei, and lysosomes, respectively, earlier than CLSM evaluation. After 1 h of incubation, each NM/siRNA NPs and MNM/siRNA NPs had been primarily localized to the cell floor. On the 6-hour mark, colocalization with lysosomes indicated endocytosis. By 12 h, MNM/siRNA NPs exhibited elevated NP alerts and lowered lysosome alerts, suggesting efficient endosomal escape, whereas NM/siRNA NPs remained colocalized with lysosomes. At 24 h, MNM/siRNA NPs alerts had been detected within the cytosol, whereas no such sign was noticed for NM/siRNA NPs (Fig. 4(d)). These findings instructed that the biomimetic neutrophil membrane successfully hindered macrophage phagocytosis, and the HA protein on the engineered neutrophil membrane prevented degradation inside endosomal compartments.
Neutrophils are well-documented for his or her recruitment to inflammatory websites by the upregulation of integrins and chemokine receptors [17, 18]. We then examined the concentrating on means of MNM/siRNA NPs to activated neutrophils at ischemic harm websites. MNHCs, stimulated by LPS to simulate activated neutrophils, had been incubated with equal concentrations of free siRNA, siRNA NPs, NM/siRNA NPs, and MNM/siRNA NPs for 4 h, utilizing Cy5-labeled siRNA. Move cytometry evaluation quantitatively revealed that LPS-stimulated neutrophils took up extra siRNA in comparison with unstimulated neutrophils (Fig. 4(e), Determine S1(e-g)). Notably, MNM/siRNA NPs handled cell confirmed a considerably increased quantity of intracellular siRNA in comparison with free siRNA, siRNA NPs, and NM/siRNA NPs handled (Fig. 4(e)). CLSM evaluation additional demonstrated the superior concentrating on functionality of MNM/siRNA NPs to activated neutrophils (Fig. 4(f)). These findings confirmed the improved concentrating on means of MNM/siRNA NPs, in all probability because of the elevated expression of integrins on the MNM, which enhanced uptake by neutrophils.
Immune escape and concentrating on means of MNM/siRNA NPs in vitro. (a) MNHC viability at totally different concentrations of siRNA NPs and MNM/siRNA NPs after incubation for twenty-four h measured by CCK-8 (imply ± SD, n = 5). (b) Consultant CLSM photographs displaying the uptake of free siRNA, siRNA NPs, NM/siRNA NPs, and MNM/siRNA NPs by RAW 264.7 cell after 4 h of incubation. Nuclei (blue), siRNA (pink); scale bar = 20 μm. (c) The uptake of free siRNA, siRNA NPs, NM/siRNA NPs, and MNM/siRNA NPs by RAW 264.7 cells after 4 h of incubation confirmed by circulation cytometry evaluation utilizing Cy5 labeled siRNA (imply ± SD, n = 5). (d) Consultant CLSM photographs of MNHCs incubated with NM/siRNA NPs, and MNM/siRNA NPs for 1, 6, 12, and 24 h. Nuclei (blue), lysosomes (pink), siRNA (inexperienced); scale bar = 10 μm. (e) The uptake of free siRNA, siRNA NPs, NM/siRNA NPs, and MNM/siRNA NPs by MNHCs after 4 h of incubation with out LPS or with LPS confirmed by circulation cytometry evaluation utilizing Cy5 labeled siRNA (imply ± SD, n = 5). (f) Consultant CLSM photographs displaying the uptake of free siRNA, siRNA NPs, NM/siRNA NPs, and MNM/siRNA NPs by MNHCs after 4 h of incubation. Nuclei (blue), siRNA (pink); scale bar = 20 μm. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (one-way ANOVA with Dunnett’s a number of comparisons take a look at)
Moreover, we quantitatively in contrast the proposed MNM/siRNA NP supply system with the siRNA transfection reagent riboFECT™CP by way of immune evasion, supply effectivity, and bioavailability (Determine S2). We discovered that the uptake of riboFECT™CP/siRNA by RAW 264.7 cells was much like that of siRNA NPs however barely increased than that of NM/siRNA NPs and MNM/siRNA NPs (Determine S2(a, b)), suggesting that the NM/siRNA NPs and MNM/siRNA NPs exhibited enhanced immune evasion properties in comparison with riboFECT™CP. Moreover, in LPS-stimulated MNHCs, the uptake of NM/siRNA NPs and MNM/siRNA NPs was considerably better than that of free siRNA, riboFECT™CP/siRNA, and siRNA NPs after 4 h of incubation (Determine S2(c, d)). This end result highlighted the superior supply effectivity and bioavailability of NM/siRNA NPs and MNM/siRNA NPs, probably as a result of their particular floor modifications and the inherent properties of their nanostructure.
MNM/siRNA NPs inhibited the formation of NETs and launch of inflammatory elements in vitro
Earlier proof signifies that integrin α9 performs a vital position within the formation of NETs and neutrophil-mediated irritation [4, 5]. Consequently, a discount in integrin α9 expression can considerably inhibit the inflammatory response. Optimum suppression of integrin α9 expression was achieved with a pABOLs/siRNA ratio of two:1 (w/w) (Fig. 5(a)). The efficacy of MNM/siRNA NPs in siRNA-mediated knockdown of integrin α9 was subsequently evaluated in vitro utilizing WB and qPCR. MNHCs stimulated with LPS to simulate activated neutrophils had been incubated with free siRNA, siRNA NPs, NM/siRNA NPs, and MNM/siRNA NPs for twenty-four h. Each NM/siRNA NPs and MNM/siRNA NPs considerably lowered integrin α9 expression in comparison with the management, free siRNA, and siRNA NPs teams (Fig. 5(b, c) and Determine S3(a)). Notably, the MNM/siRNA NPs group exhibited the bottom integrin α9 expression, indicating that the engineered neutrophil membrane on the floor of siRNA NPs enhanced efficacy.
The anti-NET impact of MNM/siRNA NPs was evaluated in MNHCs utilizing WB and enzyme-linked immunosorbent assay (ELISA). Myeloperoxidase (MPO) and citrullinated histone H3 (CitH3), as fundamental parts of NETs, together with peptidylarginine deiminase 4 (PAD4), referred to as a key regulator of NET formation [22, 23], had been each assessed. MNM/siRNA NPs therapy demonstrated a discount in NET launch in vitro, confirmed by WB evaluation of PAD4, MPO, and CitH3 (Fig. 5(d-g)). Moreover, the degrees of MPO-DNA, neutrophil elastase (NE)-DNA, and cathepsin G, all markers of NETs, had been considerably decrease within the MNM/siRNA NPs group than these of different teams (Fig. 5(h-j)), additional confirming the anti-NET results of MNM/siRNA NPs.
A number of cytokines, together with tumor necrosis issue α (TNF-α), interleukin (IL)-1β, and IL-6 secreted by neutrophils, are concerned in tissue harm induced by ischemia [24, 25]. On this research, we investigated the flexibility of MNM/siRNA NPs to neutralize inflammatory elements in vitro utilizing receptors inherited from activated MNHCs, as assessed by ELISA. The outcomes confirmed that MNM/siRNA NPs successfully neutralized the cytokines together with TNF-α, IL-1β, and IL-6 (Fig. 5(k-m)). Total, these findings instructed that MNM/siRNA NPs considerably inhibited the formation of NETs and the discharge of inflammatory elements in vitro, which may very well be attributed to the physiological perform of the specialised protein HA and integrins on the modified membrane.
MNM/siRNA NPs inhibited the formation of NETs and launch of inflammatory elements in vitro. (a) Efficient suppression of various pABOLs/siRNA weight ratios starting from 1 to 60, quantified by qPCR. (b) Consultant immune blots and (c) the corresponding quantification of integrin α9 on MNHCs after 24 h of LPS stimulation (imply ± SD, n = 5). (e) Consultant immune blots and (d, f, g) the corresponding quantification of NETs related proteins in MNHCs after 24 h of LPS stimulation (imply ± SD, n = 5). (h–j) Quantification evaluation of NET markers (MPO-DNA, NE-DNA, and cathepsin G) from the supernatant of MNHCs after 24 h of LPS stimulation measured by ELISA (imply ± SD, n = 5). (ok–m) Quantification evaluation of the inflammatory markers (TNF-α, IL-1β, and IL-6) from the supernatant of MNHCs after 24 h of LPS stimulation measured by ELISA (imply ± SD, n = 5). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (one-way ANOVA with Dunnett’s a number of comparisons take a look at)
Focusing on means of MNM/siRNA NPs in vivo
The circulation time of NPs is crucial for focused drug supply, as extended circulation can improve their recruitment to the supposed organ [26]. To evaluate the circulation time of MNM/siRNA NPs, pharmacokinetic research had been performed by measuring the fluorescence depth of Cy5-labeled siRNA at varied time factors (6, 12, 18, and 24 h) from blood and coronary heart (Fig. 6(a)). We discovered that MNM/siRNA NPs each had a stronger fluorescence depth in any respect time factors in comparison with free siRNA, siRNA NPs, and NM/siRNA NPs (Fig. 6(b-d)). These findings demonstrated that siRNA NPs, with assistance from MNM parts, exhibited a stronger sustained launch and lengthy circulation capabilities in blood and coronary heart, making them excellent carriers for siRNA supply.
Investigating potential off-target results is crucial for assessing the protection and specificity of the siRNA supply system. To additional consider the concentrating on effectivity of MNM/siRNA NPs in a mouse mannequin of MIRI, equal doses (5 mg kg− 1) of PBS, free siRNA, siRNA NPs, NM/siRNA NPs, and MNM/siRNA NPs had been administered to MIRI mice, with Cy5-labeled siRNA used for monitoring. Utilizing an in vivo imaging system, fluorescence alerts had been detected in main organs, together with the center, liver, spleen, lungs, and kidneys, 24 h post-injection. Fluorescence alerts had been detected in non-target organs such because the liver and spleen, probably reflecting the pure biodistribution and clearance pathways of NPs. Nonetheless, these alerts didn’t essentially correlate with purposeful off-target results. Quantitative evaluation revealed that the fluorescence depth within the MNM/siRNA NPs group was increased within the coronary heart in comparison with the free siRNA, siRNA NPs, and NM/siRNA NPs teams (Fig. 6(e, f)). Nonetheless, the fluorescence depth within the MNM/siRNA NPs group was decrease within the liver, lungs, and kidneys in comparison with these different teams. This enhanced concentrating on within the coronary heart is probably going because of the presence of MNM proteins on the NPs.
Focusing on means of MNM/siRNA NPs in vivo. (a) Schematic illustration of fluorescence in vivo imaging. (b) Circulation time of PBS, free siRNA, siRNA NPs, NM/siRNA NPs, and MNM/siRNA NPs with Cy5-labeled siRNA after intravenous injection in mice (n = 3). (c) Fluorescence quantification of hearts from MIRI mice at varied intervals after intravenous injection of PBS, free siRNA, siRNA NPs, NM/siRNA NPs, and MNM/siRNA NPs with Cy5-labeled siRNA (n = 3). (d) Fluorescence in vivo photographs of MIRI mice at varied intervals after intravenous injection of PBS, free siRNA, siRNA NPs, NM/siRNA NPs, and MNM/siRNA NPs with Cy5-labeled siRNA. (e) Fluorescence quantification and (f) corresponding in vivo photographs of main organs 24 h after intravenous injection of PBS, free siRNA, siRNA NPs, NM/siRNA NPs, and MNM/siRNA NPs with Cy5-labeled siRNA (n = 3)
Diminished NET formation and irritation by MNM/siRNA NPs in MIRI mice
To judge the in vivo efficacy of MNM/siRNA NPs in pulling down integrin α9, we performed qPCR and WB analyses utilizing a MIRI mouse mannequin (Fig. 7(a)). The outcomes demonstrated that each NM/siRNA NPs and MNM/siRNA NPs considerably lowered integrin α9 expression in comparison with the management, free siRNA, and siRNA NPs teams 24 h after MIRI (Fig. 7(b-d)). Notably, the MNM/siRNA NPs group exhibited essentially the most pronounced discount in integrin α9 expression, highlighting the improved efficacy offered by the engineered neutrophil membrane-camouflaged siRNA NPs.
MNM/siRNA NPs administration lowered integrin α9 expression and neutrophil infiltration in vivo. (a) Schematic illustration of the intervention course of. (b)The mRNA ranges of integrin α9 within the hearts of mice 24 h after MIRI measured by qPCR (imply ± SD, n = 5). (c) The consultant immune blots and (d) the corresponding quantification of integrin α9 within the hearts of mice 24 h after MIRI (imply ± SD, n = 5). (e) Consultant immunohistochemistry photographs for MPO + neutrophils and (f) the corresponding quantification of MPO + neutrophils within the hearts of mice 24 h after MIRI (imply ± SD, n = 5); scale bar = 100 μm. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (one-way ANOVA with Dunnett’s a number of comparisons take a look at)
The inflammatory response is a pivotal pathological mechanism in MIRI. The elevated activated neutrophils and NET formation within the coronary heart had been noticed 24 h after MIRI [27, 28]. To our delight, the neutrophil infiltration in ischemic cardiac tissue 24 h after MIRI within the MNM/siRNA NPs group was considerably lowered, as confirmed by the immunohistochemistry and hematoxylin-eosin (H&E) staining (Fig. 7(e, f), and Determine S3(b)). Furthermore, genes related to neutrophil chemotaxis and infiltration (CCR2, CXCR2, matrix metalloproteinase-2(MMP2), and MMP9) had been downregulated on this group (Determine S3(c-f)).
Moreover, the MNM/siRNA NPs group exhibited lowered ranges of PAD4, MPO, and CitH3 within the injured myocardium 24 h after MIRI (Fig. 8(a-d)), indicating a discount in NET launch in these mice. Immunofluorescence analyses additional confirmed the suppressed NET launch by MNM/siRNA NPs in MIRI mice (Fig. 8(e, f)). Furthermore, MNM/siRNA NPs considerably lowered the discharge of MPO-DNA, NE-DNA, and cathepsin G (Fig. 8(g-i)), additional validating their anti-NET results in vivo. Not like standard therapies that focus on particular cytokines with single antibodies, MNM/siRNA NPs functioned as decoys by interacting with MNM receptors to inhibit cytokine exercise and neutrophil recruitment. This method resulted in important reductions in cytokines resembling TNF-α, IL-1β, and IL-6, as demonstrated by in vivo research (Determine S3(g-i)). Collectively, MNM/siRNA NPs with their enhanced sustained launch and extended circulation capabilities, considerably lowered the inflammatory response in a mouse mannequin of MIRI by inhibiting neutrophil infiltration and NET formation.
MNM/siRNA NPs administration lowered the formation of NETs in vivo. (a) Consultant immune blots and (b–d) the corresponding quantification of NETs related to protein within the hearts of mice 24 h after MIRI (imply ± SD, n = 5). (e) Consultant CLSM photographs and (f) the corresponding quantification of NET formation within the hearts of mice 24 h after MIRI (imply ± SD, n = 5). Nuclei (blue), MPO (inexperienced), CitH3 (pink); scale bar = 20 μm. (g–i) Quantification evaluation of NET markers (MPO-DNA, NE-DNA, and cathepsin G) within the serum of mice 24 h after MIRI measured by ELISA (imply ± SD, n = 5). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (one-way ANOVA with Dunnett’s a number of comparisons take a look at)
MNM/siRNA NPs suppressed the microthrombus formation and endothelial injury in vivo
Current proof implicates that NETs work together with platelets and pink blood cells to kind microthrombus [29, 30]. Right here, we assessed thrombus density utilizing CLSM with anti-thrombocyte and anti-CD31 antibodies. Our outcomes confirmed that the administration of NM/siRNA NPs and MNM/siRNA NPs notably lowered microthrombus within the ischemic border zone (Fig. 9(a, b)). FITC-dextran staining additional confirmed improved perfusion within the injured myocardium in these teams (Fig. 9(c, d)). Furthermore, there have been no important variations in coagulation perform, together with prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT), fibrinogen, and platelet counts, between the management and intervention teams (Determine S4(a-e)). As well as, we employed CLSM with anti-albumin and anti-CD31 antibodies to evaluate vascular integrity. MNM/siRNA NPs had been discovered to protect microcirculatory integrity. Notably, much less albumin leakage was noticed within the NM/siRNA NPs and MNM/siRNA NPs teams in comparison with the management, free siRNA, and siRNA NPs teams (Fig. 9(e-f)). Moreover, we utilized WB evaluation with an anti- zonula occludens-1 (ZO-1) antibody to judge endothelial connectivity, which revealed a corresponding discount in ZO-1 ranges within the NM/siRNA NPs and MNM/siRNA NPs teams (Determine S5(a, b)). Taken collectively, these findings compellingly demonstrated the superior efficacy of MNM/siRNA NPs in mitigating microthrombus formation and endothelial injury, highlighting their potential as a promising therapeutic technique for ischemic coronary heart situations.
MNM/siRNA NPs administration suppressed the formation of microthrombus and lowered endothelial injury. (a) Consultant CLSM photographs and (b) corresponding quantification of thrombosis within the hearts of mice 24 h after MIRI (imply ± SD, n = 5). Nuclei (blue), CD31 (pink), thrombocyte(inexperienced); scale bar = 20 μm. (c) Consultant CLSM photographs and (d) corresponding quantification of FITC-dextran staining within the hearts of mice 24 h after MIRI (imply ± SD, n = 5). Nuclei (blue), CD31 (pink), FITC-dextran (inexperienced); scale bar = 20 μm. (e) Consultant CLSM photographs and (f) corresponding quantification of endothelial injury within the hearts of mice 24 h after MIRI (imply ± SD, n = 5). Nuclei (blue), CD31 (pink), albumin (inexperienced); scale bar = 20 μm. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (one-way ANOVA with Dunnett’s a number of comparisons take a look at)
MNM/siRNA NPs decreased myocardial infarction space and improved cardiac perform in vivo
To additional validate the efficacy of MNM/siRNA NPs, a mouse mannequin of MIRI was utilized (Fig. 10(a)). Mice had been administered PBS, free siRNA, siRNA NPs, NM/siRNA NPs, or MNM/siRNA NPs, and randomly divided into short-term (3–7 days) and long-term (14 days) teams. The survival price of mice within the MNM/siRNA NPs group post-MIRI was increased than that within the management, free siRNA, siRNA NPs and NM/siRNA NPs teams (Determine S6), indicating that neutrophil membrane encapsulation lowered mortality in each short-term and long-term eventualities. Notably, the MNM/siRNA NPs group exhibited the bottom mortality price, suggesting that the engineered neutrophil membrane enhanced the therapeutic impact of the siRNA NPs.
By lowering NET and microthrombus formation, MNM/siRNA NPs might considerably lower myocardial infarction space and enhance cardiac perform following MIRI. In a short-term evaluation, the infarction dimension (IS) to space in danger (AAR) ratio was considerably smaller within the MNM/siRNA NPs group in comparison with the management, free siRNA, siRNA NPs and NM/siRNA NPs teams, which was evaluated 24 h after MIRI utilizing Evan’s blue and triphenyltetrazolium chloride (TTC) staining (Fig. 10(b-d)). In a long-term analysis, myocardial fibrosis was assessed 14 days post-MIRI utilizing Masson’s trichrome staining, which revealed lowered myocardial fibrosis within the MNM/siRNA NPs group in comparison with the management and different intervention teams (Fig. 10(e, f)). Echocardiographic evaluation additional demonstrated that MNM/siRNA NPs considerably improved cardiac perform, as evidenced by elevated left ventricular ejection fraction (LVEF%) and fractional shortening (FS%), together with decreased left ventricle end-diastolic diameter (LVEDD) and left ventricle end-systolic diameter (LVESD) (Fig. 10(g-k)). These findings instructed that MNM/siRNA NPs might successfully mitigate myocardial infarction space, enhance cardiac perform, and delay the cardiac transforming course of, with the modified membrane construction contributing to enhanced preservation of cardiac perform.
MNM/siRNA NPs administration attenuated MIRI and improved cardiac perform. (a) Schematic illustration of the intervention course of. (b) Consultant photographs of Evan’s blue and TTC stained hearts from mice 24 h after MIRI. (c,d) Quantification of the proportion of AAR and IS in hearts from mice 24 h after MIRI (imply ± SD, n = 5). (e) Consultant photographs of Masson’s trichrome stained hearts from mice 14 days after MIRI; scale bar: 500 μm (high) and 50 μm (backside). (f) Quantification of the proportion of myocardial fibrosis in hearts from mice 14 days after MIRI (imply ± SD, n = 5). (g) Consultant photographs of M-mode echocardiography assessing the cardiac perform in mice 14 days after MIRI. (h-k) Quantification of LVEF, LV-FS, LVEDD, and LVESD in hearts from mice 14 days after MIRI (imply ± SD, n = 5). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (one-way ANOVA with Dunnett’s a number of comparisons take a look at)
Organic security analysis
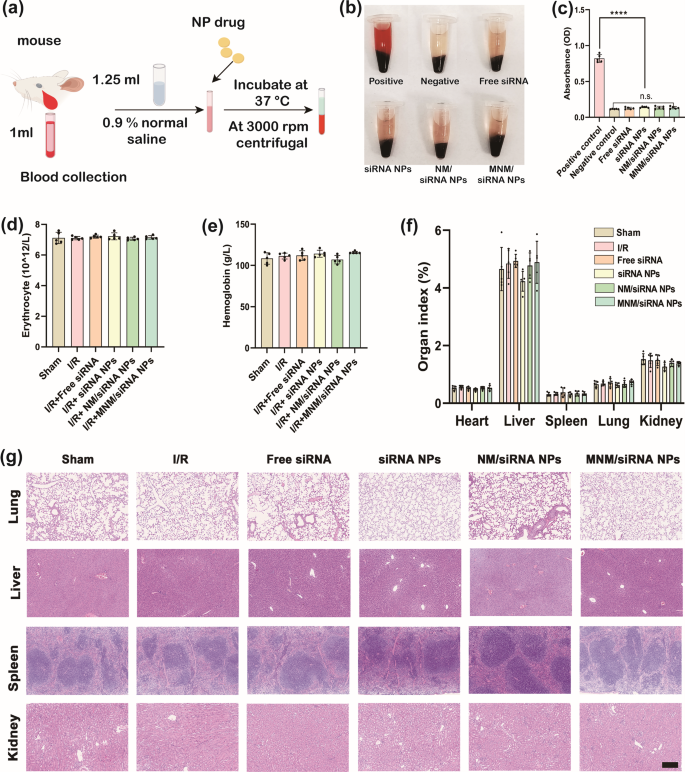
Given the potential hemolytic danger related to drug supply methods, evaluating the biocompatibility of NPs is essential. Blood compatibility, a key parameter on this evaluation, was decided through a hemolysis assay. Free siRNA, siRNA NPs, NM/siRNA NPs, and MNM/siRNA NPs had been incubated with blood utilizing a direct contact method. The absence of irregularities or particulate sedimentation within the supernatant indicated no erythrocyte injury for all NPs (Fig. 11(a-e)).
To evaluate the potential opposed results of NPs on main organs, together with the lungs, liver, spleen, and kidneys, we evaluated organ edema and harm. The organ-to-body weight ratio was measured to find out main organ edema, and H&E staining was carried out to detect tissue harm. No opposed immune responses had been noticed after the administration of MNM/siRNA NPs. Outcomes confirmed no important variations in main organ edema or harm between the management and intervention teams (Fig. 11(f, g)). Moreover, biochemical markers of liver and kidney perform, together with alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), and creatinine (CREA), had been monitored to evaluate potential hepatic and renal impairment. All marker ranges remained throughout the regular vary, indicating that the NP administration didn’t have an effect on liver or kidney perform (Determine S7(a-d)).
Organic security analysis. (a) Schematic diagram of hemolysis take a look at. (b) The consultant photographs and (c) the optical absorbance at 540 nm of the blood samples incubated with free siRNA, siRNA NPs, NM/siRNA NPs, and MNM/siRNA NPs at 37 °C for 1 h (n = 5, imply ± SD). (d,e) The erythrocyte depend and hemoglobin ranges within the mice 24 h after MIRI (n = 5, imply ± SD). (f) The organ-to-body weight ratios of mice 14 days after MIRI (n = 5, imply ± SD). (g) Consultant photographs of H&E staining of the lungs, liver, spleen and kidneys within the mice 14 days after MIRI; scale bar = 250 μm. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (one-way ANOVA with Dunnett’s a number of comparisons take a look at)