Nanoformulations
Liposomal nanoparticles
Liposomal nanoparticles, composed of a phospholipid bilayer, are nanodrug supply programs that, when administered orally, can enhance the absorption of protein medication akin to insulin. In addition they have the potential to cut back enzymatic breakdown and immune response activation, whereas being appropriate with dwelling organisms [10]. Two forms of liposomal nanoparticles have been documented: hepatic-directed vesicle insulin (HDV-I) and distearylphosphatidylethanolamine-PEG4300-folic acid (DSPE-PEG3400-FA) liposomes. HDV-I is an progressive experimental supply system comprising insulin and a hepatocyte-targeting molecule embedded in a phospholipid bilayer [11]. On this formulation, the complete quantity of insulin is loaded into the HDV to forestall its breakdown by proteolytic enzymes within the higher GI tract, thus enhancing insulin absorption. As well as, hepatocyte-targeting molecule allow the replication of regular physiological insulin administration by particularly focusing on hepatocytes. The DSPE-PEG3400-FA liposome formulation incorporates polyethylene glycol (PEG) molecules and folic acid ligands to boost the soundness of the nanoliposomes, hinder mucus penetration, and cut back mobile absorption [12]. Hydrogenated soy phosphatidylcholine was used as a thermally resistant phospholipid within the liposomes, to boost their sturdiness inside the GI system.
At the moment, there’s a lack of research analyzing the impact of oral HDV-I on animals. Researchers have developed a 5-unit HDV-I dimension two capsule. The soundness of this stable oral dosage kind has been assessed at temperatures of 5 °C, 25 °C, and 40 °C for 5 months beneath low pH circumstances, and in blood. Moreover, the small dimension of this formulation, with diameter decrease than 150 nm, has been proven to render it proof against enzymatic destruction [11, 13]. In a examine by Yazdi, et al. [12] performed to analyze the antidiabetic results of DSPE-PEG3400-FA liposomes. The diabetic rat group receiving SC insulin, confirmed probably the most important lower in blood glucose ranges inside the preliminary 2 h, with a peak occurring 1–2 h after injection. Administration of mPEG2000-DSPE-liposome-folic acid, then again, resulted in a higher discount in glucose ranges within the diabetic rats than within the rats receiving subcutaneous insulin, as noticed explicitly on the 4 h mark after administration of the formulation. With respect to the pharmacokinetic traits, the focus of insulin within the bloodstream of rats peaked 1–2 h after SC injection. In distinction, 1% and a couple of% mPEG2000-DSPE-liposome-folic acid achieved their highest ranges at 4 h after oral administration [12].
Metallic nanoparticles
Metallic nanoparticles encompass a steel core usually enveloped by a shell of an natural materials, an inorganic materials, or a steel oxide. This evaluation discusses two forms of metallic nanoparticles: gold and selenium. Gold nanoparticles are biocompatible, non-toxic, and have a excessive affinity for a lot of biomolecules, together with insulin- and protein-based constructions [14, 15]. Selenium (Se), a hint ingredient nutrient, can inhibit the event of metabolic issues, akin to T2DM, by reducing the oxidative stress [16]. There’s a shortage of analysis on using Se nanoparticles for the supply of oral insulin to provide a hypoglycemic impact equal to that of typical SC administration of insulin mimetics [17].
In 2005, a examine examined using gold nanoparticles for the supply of oral insulin. Two forms of nanoparticles had been orally administered to diabetic Wistar rats: Au-Ins and Au-Asp-Ins, through which insulin was loaded via covalent linkages and hydrogen bonding, respectively. Rats that acquired Au-Asp-Ins skilled a most lower in blood glucose ranges of 31% at 3 h after supply, whereas those who acquired Au-Ins skilled a 19% lower. The effectiveness of the Au-Asp-Ins formulation was decrease than that of the standard SC insulin (at a dosage of 5 IU/kg), the place the SC insulin demonstrated important lower of 53% in blood glucose ranges in diabetic rats, inside 2 h after supply. The Au-Asp-Ins formulation launched insulin extra quickly than Au-Ins, which might be attributed to the decrease power of the hydrogen bonding [18].
Insulin-loaded selenium nanoparticles (INS-SeNPs) had been formulated utilizing the ionic cross-linking/in situ discount methodology. The compound sodium selenite (Na2SeO3) was launched to a posh fashioned by insulin and chitosan. This was adopted by the addition of lowered L-glutathione (GSH), which precipitated Se4+ to transform to Se at its actual location, making a stable substance. Upon oral administration of three completely different doses of INS-SeNPs (12.5, 25, or 50 IU/kg) to regular Sprague–Dawley and T2DM Goto-Kakizaki rats, the dosage of fifty IU/kg had a big hypoglycemic impact in regular rats, much like that of SC insulin (1 IU/kg). This demonstrates that INS-SeNPs can resist degradation by digestive enzymes and improve insulin absorption. The numerous 21% discount in blood glucose ranges in rats handled with clean SeNPs, in comparison with ~ 20—45 of INS-SeNPs with insulin dose of 12.5, 25, or 50 IU/kg, demonstrated the antidiabetic properties of Se, which additionally contributes to this impact. In diabetic rats, the hypoglycemic impact of INS-SeNPs was dose-dependent, with a big and long-lasting impact noticed on the lowest dosage of 12.5 IU/kg. The pharmacological bioavailability confirmed a discount because the dose elevated within the order 12.5, 25, and 50 IU/kg, with values of 9.15%, 6.75%, and 4.12%, respectively. Based mostly on the every day dosage in rat research, it’s estimated that the equal Se consumption from INS-SeNPs in people is 2 mg/day. No poisonous results are believed to be related to Se within the human physique, and Se has not been reported to have an effect on the human physique, both genetically or immunologically [19].
The abstract of the metallic nanoparticle formulation is demonstrated in Desk 1.
Polymeric nanoparticles
Polymeric nanoparticles are small particles able to encapsulating energetic substances, both by trapping them inside the polymeric core or absorbing them onto their floor. Many of those nanoparticles encompass biopolymers, akin to albumin, collagen, gelatin, keratin, and silk proteins; or polysaccharides, akin to alginate and chitosan [20]. These biopolymers are biocompatible, making them each biodegradable and non-toxic, even after extended publicity. The polymeric nanoparticles talked about under have been categorised into single-stage or multi-stage formulations based mostly on the extent of complexity of their preparation.
Single-stage polymeric nanoparticles
Chitosan is a biocompatible polymer ceaselessly used for drug supply. A examine performed by Sonaje, et al. [21] utilized pH-responsive nanoparticles composed of chitosan and poly(γ-glutamic acid) to manage insulin aspart (a speedy performing insulin), orally. A transepithelial electrical resistance check subsequently carried out on Caco-2 cells confirmed that the cationic chitosan nanoparticles quickly broke the sturdy bonds between the cells. One other examine examined the pharmacodynamics of oral insulin aspart, SC insulin aspart, regular insulin, impartial protamine Hagedorn (NPH) insulin (an intermediate-acting insulin), and insulin detemir (a long-acting insulin). The pharmacokinetic investigation concerned a comparability of SC administration of insulin aspart and NPH insulin. In a rat mannequin of diabetes, SC administration of insulin aspart or regular insulin resulted in a pronounced discount in blood glucose ranges over a relatively shorter time frame (2–3 h). Conversely, when insulin aspart-loaded nanoparticles had been administered orally, there was a noticeable however slower lower in blood glucose ranges inside 6 h of supply in comparison with SC insulin, adopted by a gradual improve. Insulin aspart exhibited enhanced absorption kinetics when administered by the SC route, with the utmost plasma focus (Cmax) achieved 30 min following oral administration, as in comparison with 3 h post-injection by the oral route. Oral administration of insulin aspart reduces the probability of hyperinsulinemia, a situation characterised by extreme insulin ranges, as it’s absorbed step by step and repeatedly over an prolonged length, with out inflicting speedy spikes. Oral insulin aspart has the potential for use as an alternative of basal insulin remedy based mostly on its pharmacokinetic profile, which is analogous to that of subcutaneous NPH insulin. The nanoparticles used to ship oral insulin aspart had a relative bioavailability of 15% [21].
Thiolated chitosan nanoparticles (TCNPs) loaded with insulin are one other kind of polymeric nanoparticles based mostly on chitosan which can be presently beneath investigation. Prolonged insulin launch from TCNPs has been reported in vitro, at pH 5.3. When diabetic rats handled with streptozotocin had been orally administered insulin-loaded TCNPs (Ins-TCNPs), their blood glucose ranges decreased extra slowly and the quantity of insulin of their blood elevated in comparison with when insulin was injected through the SC route. The prolonged length of insulin motion is probably going attributable to the interplay between the thiol group and glycoproteins within the intestinal mucus. Evaluation of the biocompatibility of TCNPs in Caco-2 cells revealed that the cell viability remained unchanged at TCNP concentrations under 1000 μg/mL, indicating no substantial impression. Nevertheless, the viability of the cells steadily declined because the focus of TCNPs rose above 1000 μg/mL [22].
The usage of trimethylchitosan (TMC) nanoparticles to orally ship insulin was investigated by conjugating them to glycyl-glycine (GG) and alanyl-alanine (AA). Earlier research have indicated that conjugating polymers with dipeptides can enhance their absorption throughout enterocytes by permitting them to move via the intestinal layer utilizing the proton-coupled oligopeptide transporters PepT1 and/or PepT2. Characterization of the nanoparticles for subsequent experiments decided {that a} focus of 1.5 mg/mL for each polymer conjugates and insulin focus was most popular for the particle fabrication. An experiment performed in rats with diabetes demonstrated that oral administration of trimethylchitosan-carboxymethyl-glycyl-glycine (TMC-CM-GG) and -alanyl-alanine (TMC-CM-AA) nanoparticles lowered the focus of glucose within the blood over a interval of 8 h after supply. The lower in serum glucose focus following the oral administration of TMC-CM-GG was much like that noticed after SC insulin administration, with no important distinction. Particularly, the blood glucose stage was lowered by 46.8% of the preliminary stage at 8 h after oral administration, in comparison with the noticed discount of 64.4% at 2 h after SC administration. Conversely, ingestion of TMC-CM-AA nanoparticles resulted in a discount of 54.9% within the focus of glucose within the bloodstream at 8 h, as in comparison with ingestion of insulin resolution, which confirmed no impact. The serum insulin ranges attained by dipeptide conjugation had been roughly half of these attained by SC insulin injection. The relative bioavailability was 17.19% for TMC-CM-GG and 15.46% for TMC-CM-AA. Within the toxicity exams, the addition of all polymer conjugates to Caco-2 cells led to survival charges of > 65% and > 50% at focus of 1 and 5 mg/mL, respectively. As compared, the controls, that are insulin options with focus of 1 and 5 mg/mL, exhibited survival charges of 87.3% and 76.1%, respectively [23].
Moreover, researchers are analyzing the potential of poly(isobutylcyanoacrylate) as a polymeric drug provider owing to its stability and skill to interrupt down naturally within the human physique. When remodeled into nanocapsules, their small dimension (< 300 nm in diameter) permits for absorption within the intestines [24, 25]. Thus, it was hypothesized that using poly(isobutylcyanoacrylate) nanocapsules enhances the assimilation of insulin. Damgé, et al. [26] confirmed that poly(isobutylcyanoacrylate) nanocapsules successfully lowered blood glucose ranges in streptozotocin-induced diabetic fasting rats, inside 2 d of oral administration. Therefore, a more moderen investigation examined the extent to which insulin might be absorbed into the bloodstream following the ingestion of poly(isobutylcyanoacrylate) nanocapsules. The nanocapsules had been created by way of interfacial polymerization utilizing isobutylcyanoacrylate. These findings demonstrated that oral administration of insulin-loaded nanocapsules on the dose of fifty mIU/kg successfully transported insulin into the systemic circulation, as evidenced by a big improve in blood insulin ranges within the diabetic rats. The measured plasma insulin ranges ranged from 50 to 240 mIU/L. The insulin focus within the blood of the rats diverse, with the rats falling into two distinct classes: these with excessive absorption and people with low absorption. Though the insulin stage elevated, there was no detectable drop in blood glucose ranges even 2 d after the supply of the nanocapsules, which contradicts the findings of earlier research. When insulin was administered to rats via injection at a dose of 5 IU/kg, blood insulin ranges elevated to 5500 mIU/L in regular rats and 4000 mIU/L in diabetic rats. Nevertheless, the conventional rats confirmed a higher lower in blood sugar ranges than the diabetic rats. This means that the dearth of hypoglycemic impact in rats handled with orally delivered insulin-loaded nanocapsules could also be as a consequence of insulin resistance. Insulin resistance in rats requires excessive insulin concentrations within the bloodstream to exert an impression [27].
The hypoglycemic results of insulin-containing Eudragit RS-100 (ERS-100) nanoparticles have been studied in diabetic rabbits and sheep. In response to Olya, et al. [28] and Trapani, et al. [29], when taken orally, the polymeric system ERS-100 shields the peptides it comprises from denaturation. ERS-100 doesn’t dissolve at physiological pH however swells when added to water, which prevents enzymes from breaking it down in enteric formulations [30]. In vivo research utilizing diabetic rabbits discovered that gavage administration of ERS-100 nanoparticles containing insulin (ILNP) considerably lowered blood glucose ranges by 40 mg/dL and maintained this discount for two d. Moreover, a examine in sheep handled with oral ILNP confirmed that the blood glucose ranges on Day 5 had been considerably decrease than these within the management group. It was additionally famous that post-treatment insulin ranges weren’t affected at any sampling time, suggesting that the pancreas may need secreted insulin via a destructive suggestions mechanism upon oral administration of ILNP. Cortisol ranges, that are identified to be related to gluconeogenesis, had been additionally considerably lowered within the ILNP-treated group, as in comparison with that within the controls [28].
ERS-100 nanoparticles have additionally been blended with poly(ε-caprolactone), a biodegradable polymer, to make insulin nanoparticles utilizing the double emulsion methodology. In an in vivo examine performed to judge the results on blood glucose ranges in diabetic rats, as in comparison with the management group, nanoparticles encapsulating 100 IU/kg insulin considerably lowered glycemia by 41% on the 4th hour post-oral administration, and this impact lasted at the very least 8 h. Equally, the world beneath the receiver working attribute curve (AUC) of blood glucose ranges lowered by 23% and 38% upon remedy with nanoparticles containing 50 and 100 IU/kg insulin, respectively, as in comparison with that within the management group. The relative bioavailability of the insulin-loaded nanoparticles was 13.21% [31].
In case of PEGylated starch acetate nanoparticles, amphiphilic PEG with a molecular weight of 1900 Da was conjugated with hydrophobic starch acetate by way of spontaneous aggregation to kind micelles with hydrophobic cores. An in vitro examine of its insulin launch profile confirmed that ~ 20% and ~ 55% of insulin was launched at pH 1.2 and seven.4, respectively, inside 2 h. Then again, after 8 h in simulated intestinal fluid with a pH of 6.8 and phosphate-buffered saline with a pH of seven.4, 60% and 80% of insulin was launched, respectively. In distinction, the noticed cumulative insulin launch of 20% after 2 h within the simulated gastric fluid was comparatively low, contemplating that this was the common gastric transit time. The sustained launch of insulin from the nanoparticles noticed at impartial or fundamental pH was achieved by diffusion or swelling of the nanoparticles, which stabilized the insulin by stopping it from self-association upon launch from the nanoparticles. Evaluation of the cytotoxicity of PEGylated starch acetate nanoparticles discovered that > 98% of the L929 cells incubated with the nanoparticles had been viable. The nanoparticle-induced hemolysis was additionally insignificant, as in comparison with that induced by poisonous PEG nanoparticles with low molecular weights (500 and 800), thereby demonstrating its security as a supply system [32].
A starch-based nanocomposite consisting of short-chain glucans (SCGs), that are debranched starches, has been investigated for its potential as an insulin supply nanocarrier. Proanthocyanidins (PACs) had been added to stabilize insulin and facilitate hydrogen bonding between SCG, insulin, and PAC. After oral administration of insulin-SCG-PAC to diabetic rats, the blood glucose ranges decreased by as much as 36.84% on the third hour and had been sustained for the complete examine interval of 8 h, in distinction to that in case of the SC insulin injection, the place the blood glucose ranges spiked again to its preliminary stage at 6 h. This may increasingly even be defined by the truth that smaller particle dimension leads to higher insulin absorption within the gut. The pharmacological exercise of insulin-SCG-PAC was 6.98%, whereas that of the oral insulin resolution was 0.64%. A cell viability check performed utilizing undifferentiated Caco-2 cells to analyze the cytotoxicity of the nanoparticles confirmed that the insulin-SCG-PAC nanocomposite was low in cytotoxicity, with relative cell viability above 90% at concentrations of 125 to 500 μg/mL and even > 85% on the larger focus examined, which was 1000 μg/mL [33].
The preclinical research on single-stage nanoparticle formulations are summarized in Desk 2.
Glucose-responsive polymeric nanoparticles
Hypoglycemia is a well known antagonistic impact of insulin remedy, with signs starting from confusion and anxiousness to potential lack of consciousness and even loss of life. Analysis has explored strategies to automate insulin supply based mostly on blood glucose ranges, together with glucose-sensitive subcutaneous insulin, which reveals promise in decreasing the incidence of hypoglycemic episodes. This strategy includes the binding of glucose to a macrocycle linked to insulin, enabling the managed launch of insulin in response to rising glucose ranges [34]. Equally, within the context of oral administration, glucose sensitivity will be achieved via redox reactions of particular excipients beneath hyperglycemic circumstances, which modulate the discharge of insulin accordingly. Zhou, et al. [35] designed a glucose-responsive supply system that releases insulin relying on the blood glucose focus. This was achieved by having practical sections like phenylboronic acid (PBA), glucose binding proteins and glucose oxidase (GOx), integrated of their particles in addition to designing a 2-nitroimidazole-l-cysteine-alginate (NI-CYS-ALG) polymer, the place every practical group performs an vital function for the supply: (1) 2-Nitroimdazole (NI): Underneath hyperglycemic circumstances, glucose oxidase (GOx) loaded into the particles alongside insulin, induces hypoxic circumstances resulting in the discount of NI to the hydrophilic 2-aminoimidazole. (2) L-Cysteine: The thiol teams work together with the cysteine-rich subdomains of mucus glycoproteins within the small gut and due to this fact prolongs the resistance time inside the GI tract and will increase the pH stability of the system. Moreover, it improves intestinal permeability because it adjustments the distribution of F-actin and ZO-1. (3) Alginate: the hydrophilic polyanion polymer has mucoadhesive properties. When incubating the particles with completely different concentrations of glucose, the scale step by step elevated from 230 to 616 nm within the first hour and to 977 nm inside 6 h. This dimension improve was because of the discount of NI within the hyperglycemic situation attributable to GOx. Moreover, in hyperglycemic resolution (400 mg/dL glucose), the particles had a excessive insulin launch ratio (30.43 ± 15.64% within the first hour and 87.56 ± 6.95% after 10 h), because of the dissociation of the particles. Particles uncovered to regular circumstances (100 mg/dL glucose), solely launched a part of the insulin (25.10% ± 5.97% inside 10 h) [35].
Upon administration to diabetic rats, each formulations, one containing co-loaded insulin and GOx, and the opposite with insulin alone, exhibited a robust hypoglycemic impact. Subcutaneous injection of insulin decreased the blood glucose stage to 12.2 ± 1.0% after 1 h, nonetheless the degrees returned to hyperglycemia after 4 h. The insulin-loaded particles managed to cut back the degrees to 45.1 ± 4.1% of the preliminary focus by 8 h and saved these ranges for an additional 6 h. Particles loaded with GOx and insulin managed to lower blood glucose ranges to 30.2 ± 1.9% for two h and will preserve euglycemic ranges as much as 12 h, show-casing the profitable design of a glucose-responsive drug supply system in a position to cut back and preserve blood glucose to euglycemic ranges [35].
Multi-stage polymeric nanoparticles
On this examine, a two-stage polymeric nanoparticle supply system was developed. The primary stage is pH-sensitive, whereas the second includes sticking to the mucosa. Collectively, these levels can overcome the limitations that make oral insulin absorption difficult [36]. First, the outer layer of the exhausting gelatin capsules was coated with hydroxypropyl methylcellulose phthalate (HP55), an enteric polymer delicate to pH, with a pKa of 5.50. This prevents oral insulin from dropping its efficacy as a consequence of enzyme breakdown and the low-pH surroundings of the abdomen. In contrast to ERS-100, HP55 dissolved within the higher a part of the small gut when the pH was larger than its pKa. This allowed the nanoparticles to flee from the capsule that breaks aside. Second, cationic nanoparticles containing insulin made from poly(lactic-co-glycolic acid) (PLGA) and ERS had been absorbed throughout the gut. Regardless of being non-biodegradable, ERS-100 is biocompatible and may adhere to the mucosal layer of the GI tract [29]. The improved absorption is probably because of the longer residence time within the gut and its cationicity, which opens tight junctions. The insulin-loaded PLGA/ERS nanoparticles had been formulated by way of ultrasonic emulsification utilizing the multiple-emulsion solvent evaporation methodology. In vitro outcomes confirmed that HP55-coated capsules lowered insulin launch at pH 1.2 from 90 to 10%. Nevertheless, PLGA/ERS nanoparticles launched insulin at pH 7.40 in the identical manner with or with out the HP55-coated capsule, suggesting that the enteric coating doesn’t change insulin launch at this pH, as demonstrated in Fig. 3 with comparability to ERS-100 nanoparticles, single-stage nanoparticles. An in vivo examine on diabetic rats confirmed that oral administration of enteric-coated capsules crammed with insulin-loaded PLGA/ERS nanoparticles induced a hypoglycemic impact of 32.9% (outlined as the world above the curve of the plasma glucose stage), at a dose of fifty IU/kg, with a time to achieve peak focus (Tmax) of 10 h. This was similar to the outcomes obtained in rats injected with SC insulin at a dosage of 5 IU/kg (35.20%), with Tmax on the 2nd hour. The supply system demonstrated a chronic hypoglycemic impact in a diabetic rat mannequin, with a pharmacological availability of 9.2%. These outcomes urged that the two-stage supply system is a possible strategy for enhancing the efficacy of oral insulin supply [36].
Comparability between single-stage and multi-stage polymeric nanoparticles. Comparability between single-stage and multi-stage polymeric nanoparticles. ERS-100 NPs and PLGA-ERS NPs in HP55 enteric-coated capsules had been in contrast on the premise of ERS-100 because the frequent excipient.
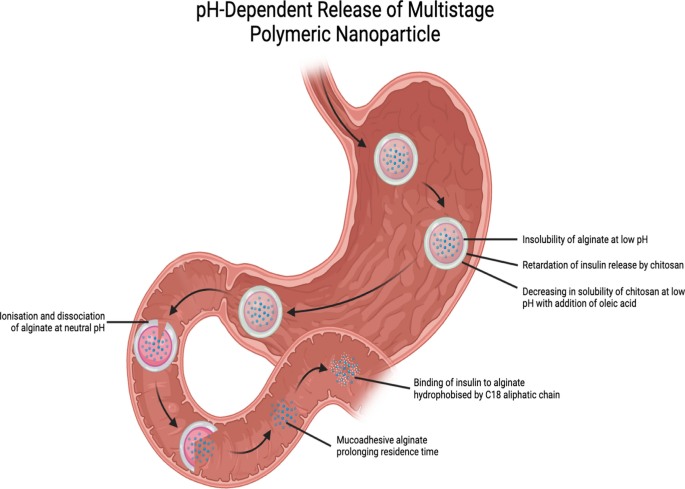
Nanoparticles in beads have additionally been investigated, particularly insulin-loaded alginate nanoparticles microencapsulated in pH-sensitive alginate beads and coated with a layer of chitosan-oleic acid. Alginate beads possess protecting properties that delay the discharge of nanoparticles into the gastric surroundings. The mucoadhesive properties exhibited by alginate following dissociation and ionization at impartial pH within the intestines additionally lengthen the residence time of the nanoparticles within the intestines. As well as, alginate can bind to dietary glucose within the intestines and impede its absorption into the bloodstream. Including oleic acid to the chitosan spine slowed the discharge of the payload. It is because chitosan doesn’t dissolve simply beneath acidic circumstances, which retains its interactions with alginate intact. Within the drug launch profile and in vivo research, alginate-C18 nanoparticles (AC18N) had been used. The addition of C-18 aliphatic chains could make alginate extra hydrophobic, stopping it from interacting with insulin and blocking its launch. Chitosan-oleic acid conjugate-coated calcium alginate beads (CCAB) loaded with AC18N launched 2.8% of the insulin loaded within the first 2 h, in simulated gastric fluid at pH 1 (Fig. 4). After transferring to simulated intestinal fluid with a pH of 6.8, 24.7% of the loaded insulin was launched over the subsequent 4 h. The AC18N-CCAB additionally precipitated a higher lower within the blood glucose ranges within the diabetic rats than that noticed within the management teams and upon remedy with SC insulin and free insulin-loaded nanoparticles. Nevertheless, an exception was noticed within the case of the pattern obtained at 30 min, probably because of the digestion of the polysaccharide content material of the formulation. This was per the blood insulin stage at 24 h, which was considerably larger within the AC18N-CCAB-treated diabetic rats than that noticed within the case of remedy with insulin-loaded AC18N. It’s value mentioning that CCAB loaded with insulin-free AC18N induced a higher discount in blood glucose ranges than that loaded with insulin-containing AC18N, owing to the sugar-binding property of alginate. Upon evaluation of the toxicity profile in HT29 cells, insulin-loaded AC18N was similar to the management, indicating low toxicity of AC18N [37].
One other potential insulin provider are self-assembled N-(2-hydroxypropyl) methacrylamide (pHPMA) polymeric nanoparticles. The nanocomplex (NC) core consists of anionic insulin blended with polycationic penetratin, a cell-penetrating peptide, whereas coating layer consists of dissociable negatively charged hydrophilic pHPMA that self-assembles upon the addition of the NC to the pHPMA resolution. pHPMA with various MA-GG-OH monomer ratios has been used to find out the molecular construction. Utilizing an Ussing Chamber System to simulate the mucus layer, epithelial cells beneath the porcine intestinal mucus, and a semipermeable membrane, it was discovered that the pHPMA coating was in a position to enhance permeation, as in comparison with that noticed in case of uncoated NCs, due to its means to curtail the interactions between the NCs and anionic/hydrophobic areas of the mucin construction within the mucus (Fig. 5). Nevertheless, it grew to become much less permeable because the cost density of pHPMA elevated, most likely because of the repulsion between pHPMA and mucus, that are each negatively charged. The power of pHPMA-coated NCs to permeate via the mucus was additional demonstrated by way of the Brownian actions noticed within the mucus and an in vitro insulin uptake examine, whereas uncoated NCs had been trapped within the mucus owing to interactions. In an in vivo examine evaluating the blood glucose levels-lowering results of saline, PO-free insulin resolution, SC insulin resolution, uncoated NCs, and pHPMA nanoparticles with the bottom cost density in diabetic rats, pHPMA nanoparticles demonstrated a maximal discount of fifty% in blood glucose ranges, at 4 h post-administration with a dosage of 75 IU/kg. Orally administered free insulin had nearly no impact on glucose ranges, whereas the NCs precipitated a slight lower. SC insulin confirmed the best discount, which peaked at 2 h post-administration; this was mirrored within the serum insulin stage as effectively, which peaked on the first hour. Serum insulin ranges in diabetic rats administered pHPMA nanoparticles peaked on the 4th hour. The pharmacological availability of the pHPMA nanoparticles was 6.61%, greater than 2 occasions that of the uncoated NCs (2.60%), validating the operate of the pHPMA coating. In vitro security analysis utilizing the HT29-MTX-E12 (E12) cell line demonstrated no important cytotoxicity over the focus vary of fifty to 200 μg/mL [38].
Enhanced permeation of self-assembled N-(2-hydroxypropyl) methacrylamide (pHPMA) polymeric nanoparticle throughout intestinal lining. Interplay between the nanocore uncoated with pHPMA and the mucin is depicted in crimson line which hinders the insulin from passing via the mucus barrier.
In a examine on the formulation of insulin-loaded alginate/dextran sulfate nanoparticles (ADS-NPs), the nanoparticles had been dual-coated with chitosan and albumin (ALB). As an anionic copolymer, dextran sulfate can enhance the loading of hydrophilic medication into alginate. Coating with ALB can forestall the proteolytic degradation of insulin by blocking protease entry and stabilizing the nanoparticles in each acidic and intestinal environments. A examine on the insulin launch profile in simulated gastric and intestinal media confirmed that dual-coated nanoparticles (ALB-NPs) may retain insulin encapsulated at pH 1.2 and burst-release insulin at pH 5.5. That is potential because of the insolubility of alginate at low pH and cross-linking between the positively charged alginate and dextran sulfate. Moreover, at pH 7.4, a sustained launch profile of insulin was noticed, as chitosan, which is soluble at a decrease pH, grew to become insoluble at this pH and will retain insulin. In an in vitro examine of the insulin permeation profile of those nanoparticles with a triple co-culture of the Caco-2/HT29 cells tailored to methotrexate (HT29-MTX) (a human colon most cancers cell-line)/Raji B (a lymphoblast-like cell-line) cell mannequin, which mimics the monolayer of the gut, the permeation of ALB-NPs was discovered to be considerably larger than that of non-encapsulated insulin. At pH 7.4, the insulin launched from the ALB-NPs was nearly 100% after 3 h, per the discharge profile. An in vitro cell viability examine utilizing AGS (a human gastric adenocarcinoma cell-line), Caco-2, and HT29-MTX cell traces incubated with completely different concentrations (0.10, 0.25, 0.50, and 1.00 mg/mL) of ALB-NPs confirmed that the nanoparticles didn’t considerably decrease the cell viability throughout all cells, as in comparison with the destructive management of HBSS-HEPES buffer, aside from on the decrease concentrations of 0.1 and 0.25 mg/mL in AGS cells [39].
A nanoparticle with an similar composition was studied for its hypoglycemic impact upon oral administration. Insulin-loaded nanoparticles had been discovered to realize sustained hypoglycemic results in diabetic rats that had been comparable between the doses of fifty and 100 IU/kg; the absence of a dose–response impact on this case might be attributed to saturation. The hypoglycemic impact achieved by each concentrations of insulin-loaded nanoparticles was considerably completely different from that of unencapsulated insulin between the eighth and twelfth h. For the biodistribution examine in diabetic rats, the place technetium-99 m-albumin (99mTc-BSA) was used for radiolabeling the nanoparticles, the radioactivity of the 99mTc-BSA nanoparticles within the abdomen wall elevated solely 60 min after oral administration after which decreased steeply within the abdomen contents, and at last subsided to < 50% after 90 min. Within the small gut wall, the radioactivity of 99mTc-BSA nanoparticles between 120 and 180 min was considerably larger than that of the management group, 99mTc-BSA, through which case the radioactivity plummeted at 120 min, exhibiting that retention was related to the interplay between the twin coating and epithelial cells [40].
The preclinical research associated to multi-stage polymeric nanoparticle formulations are summed up in Desk 3.
Porous silicon nanoparticles
Porous silicon nanoparticles have confirmed efficient as carriers for numerous medication and routes of administration. Resulting from their excessive loading capability, customizable floor chemistry, and resilience to harsh circumstances, akin to these encountered within the gastrointestinal tract, they’re perfect candidates for oral drug supply [41]. Furthermore, the restricted areas inside their pores contribute to enhancing drug solubility by stopping the formation of crystalline supplies [42].
Rao et al. [43] used these benefits by designing a virus-mimicking porous silicon nanoplatform modified with poly (pyridyl disulfide ethylene phosphate/sulfobetaine) (P(PyEP-g-SB) polymers to enhance mucus permeability and mobile internalization. By conjugating dodecyl sulfobetaine (SB) to the aspect chains of poly (pyridyl disulfide ethylene phosphate) (PPyEP) the generated degradable zwitterionic polyphosphoester resembled viral polymer molecular brushes. The zwitterionic aspect chains facilitated interplay with intestinal mucus and when the phosphoester moieties begin to degrade because of the intestinal alkaline phosphatase (IAP), the spine items and the cationic cores of the nanoparticles facilitate the mobile uptake [43].
Evaluation of mobile uptake of the functionalized particles in Caco-2 cells revealed that positively charged teams elevated mobile uptake and with growing SB items, mucus penetration and transcellular transport improved. When testing this technique in streptozotocin-induced diabetic rats, blood glucose stage didn’t cut back after the oral administration of a free insulin resolution or empty particles. After the subcutaneous injection of insulin, the blood glucose ranges dropped, however recovered after 4 h. Nevertheless, after administration of P(PyEP-g-SB0.3)20- AmPSiNPs (50 IU kg−1), the blood glucose ranges had been considerably lowered for 8 h (34.5%). The unfunctionalized AmPSiNPs confirmed much less pronounced blood glucose discount (10.3%), with the functionalized particles exhibiting a 1.38 fold larger blood insulin launch inside the first hour. Nevertheless, each teams resulted in steady ranges after 2 h, which signifies that insulin will be launched step by step from the particles. Though the hypoglycemic impact of the functionalized particles extended for as much as 8 h, the mice had been euthanized, because the extended fasting may have an effect on the hypoglycemia impact. Functionalized particles exhibited the very best relative oral bioavailability with 4.36%, compared of two.09% with free insulin and three.47% with unfunctionalized particles [43].
Quantum dots
Hunt, et al. [44] designed pH- and enzyme delicate silver sulfide (Ag2S) quantum dots (QDs) for the oral supply of insulin. In earlier works, Ag2S QDs improved the oral bioavailability of metformin and nicotinamide mononucleotide 100–10.0000-fold [45, 46]. With a purpose to shield the loaded insulin in addition to obtain managed launch, the authors designed a random polymerized chitosan and glucose copolymer (CS/GS) across the quantum dot insulin assemble, which is extremely delicate to enzymatic hydrolysis, particularly by ß-glucosidase [44].
Human duodenal explants had been live-imaged to analyze the mobile uptake of QDs within the small gut. These exams revealed that after 2–4 min, round 70% of the particles had been positioned within the cytoplasm and after 8 min, the formation of endocytic vesicles and exocytosis might be noticed. Moreover, when insulin was loaded into the QD-INS-CS/GS system, the uptake of insulin was elevated 40-fold in comparison with insulin alone. When testing the system in rats, subcutaneous injection of insulin result in a 30% improve in physique weight, in comparison with no adjustments when being handled with QD-INS-CS/GS. Moreover, no adjustments in serum biochemistry or lipids had been noticed [44].
An important discovering on this examine was the absence of hypoglycemia when the system was examined in mice, rats and baboons. By using a glucosidase-responsive system, this technique makes use of the correlation between the β-glucosidase chemical exercise and blood glucose focus, suggesting that the degradation of CS/GS depends on the glucose focus [44].
Microparticles
Microparticles, outlined as spherical particles with a dimension between 1 and 1000 μm in diameter, have additionally been studied for insulin supply. Chitosan phthalate microspheres have been used due to their pH-sensitive properties; they show low solubility at low pH and are fully soluble at fundamental pH. Insulin is efficiently entrapped in these microparticles utilizing an emulsion crosslinking method. An in vivo examine on streptozotocin-induced diabetic rats confirmed that these microspheres demonstrated a maximal lower in blood glucose ranges to 51.54% of the preliminary stage at 6 h post-administration, and the glucose-lowering impact remained important, as in comparison with that of an orally administered chitosan phthalate-insulin resolution, for at the very least 16 h post-administration. It was additionally considerably decrease than SC insulin from the sixth to twentieth hour, because the minimal glucose stage of SC insulin was achieved on the 1st hour post-administration and returned to the preliminary stage thereafter. The relative pharmacological efficacy of this formulation was 18.66%, which is roughly 4 occasions larger than that of the oral chitosan phthalate-insulin resolution (5.75%), whereas that of SC insulin was 14.82% [47].
One other type of microparticles is a microparticulate solid-in-oil-in-water emulsion, through which the enteric polymer hydroxypropyl methylcellulose phthalate is current within the aqueous section [48]. A pH-dependent launch profile was demonstrated, through which the share of insulin launched was larger at larger pH values and was additional boosted within the presence of lipase.
Hydrogels
Hydrogels are three-dimensional community constructions consisting of polymers which can be bodily or chemically crosslinked and hydrophilic, which permits them to retain water and swell with out dissolving into aqueous environments [49]. The efficacy and security profiles of various hydrogel formulations, together with polymethacrylic acid (PMAA)- and polysaccharide-based hydrogels akin to cellulose and chitosan, have been investigated to ship insulin.
The PMAA hydrogel and its variants have been studied as a result of PMAA can enhance the permeability of hydrophilic compounds throughout epithelial cells and inhibit the enzymatic motion of calcium-dependent proteases by binding to calcium ions [50, 51]. A PMAA-chitosan-PEG (PCP) hydrogel with or with out thiolation was developed, through which functionalization with a thiol group made the hydrogel mucoadhesive, thereby enhancing its retention on the mucus layer and diffusion throughout the layer. Within the case of the non-thiolized PCP hydrogel, the hydrogel loaded with insulin was complexed with methyl-β-cyclodextrin, as its hydrophobic nature can improve the absorption of hydrophilic insulin throughout intestinal cells and impede its self-association. Within the launch examine, insulin-loaded PCP demonstrated pH-sensitive properties, as solely 10% of insulin was launched inside 2 h at pH 1.2, whereas a excessive proportion of it was launched inside 3 h at pH 7.4. An in vivo examine in diabetic rats confirmed that oral administration of complicated insulin-loaded PCP lowered glycemia ranges by 30% in 2 h, which was additional sustained for six h. Regardless of the extent growing thereafter, it was nonetheless 10% decrease than that within the management group, which was not administered any remedy even after 10 h. The relative pharmacological bioavailability of the insulin-loaded PCP was 1.95. As for the security of methyl-β-cyclodextrin, its cytotoxicity in direction of Caco-2 cells was discovered to be concentration-dependent, as at a focus of 25 mM it resulted in a cell viability of 0%, however under 10 mM, it was non-cytotoxic [50].
Within the case of the thiolized variant, thiolization was carried out by way of conjugation with cysteine. In Caco-2 cells, insulin encapsulated in cysteine-conjugated PCP microparticles (Cys-PCP) was nearly 5 occasions extra permeable than the unencapsulated management, whereas unconjugated PCP confirmed thrice larger permeability than the management. Each PCP and Cys-PCP precipitated a > 50% lower in transepithelial electrical resistance throughout the monolayer of Caco-2 cells, indicating that tight junctions between cells had been loosened on account of calcium binding and tyrosine phosphatase-mediated inhibition of occluding, a transmembrane protein that’s concerned within the closing of tight junctions (Fig. 6). PCP and Cys-PCP had been 2.5- and a couple of.8-fold extra permeable throughout rat intestinal tissues than the management, respectively. In diabetic rats, Cys-PCP lowered blood glucose ranges by ~ 40% from the preliminary stage in 2 h, and this impact was sustained for over 8 h, whereas PCP lowered them by 15%, which was sustained after 6 h. The relative pharmacological availability of the Cys-PCP particles was discovered to be 2.45 [51].
PMAA polymers will also be grafted with one other polymer, PEG, to kind a copolymer hydrogel community generally known as poly(methacrylic acid-grafted poly(ethylene glycol)) [P(MAA-g-EG)] [52, 53]. Because the interactions between each polymers contain hydrogen bonding, the copolymer networks can exhibit pH-sensitive properties, the place the hydrogel swells at a better pH and collapses at a decrease pH. P(MAA-g-EG) hydrogel particles encapsulating insulin have been proven to have a good hypoglycemic profile in research on diabetic rats. Administration of 25 IU/kg insulin loaded within the (P(MAA-g-EG)) hydrogel to streptozotocin-induced Wistar diabetic rats resulted in a discount of ~ 40% within the blood glucose ranges for a interval longer than 8 h. The bioavailability of insulin ranged from 2.44% to 4.22%, relying on the encapsulated insulin dose and induced diabetic standing within the rats, as in comparison with the vary of 0.55% to 0.88% noticed in case of the management insulin resolution [52]. The dimensions and composition of hydrogel microparticles additionally impacts the oral insulin bioavailability. A examine by Morishita, et al. [53] confirmed {that a} 1:1 molar ratio of methacrylic acid to ethylene glycol was perfect for inducing the very best proportion of glucose discount, as indicated by the world above the curve, and had the very best pharmacological availability of seven.4%, as in comparison with these noticed on the ratios of 1:0 and 4:1. With respect to the scale, microparticles with diameters of < 53 µm confirmed the best proportion of glucose discount, highest pharmacological availability, and speedy launch of insulin, as in comparison with microparticles with diameters between 212 and 300 µm [53].
One other instance of a copolymeric hydrogel is a hydrogel community composed of succinyl-chitosan-grafted polyacrylamide. Whereas addition of a succinyl group to chitosan improves its hydrophilicity and pH sensitivity, polyacrylamide grafting can improve the variety of encapsulated insulin molecules by introducing extra amino teams into the hydrogel. The S-chitosan-grafted polyacrylamide (PAA/S-chitosan) hydrogel was discovered to launch 20%–25% insulin beneath simulated gastric circumstances, at pH 1.2, and ~ 98% insulin within the simulated intestinal fluid. This may be attributed to the protonation standing of the carboxyl group on S-chitosan at completely different pH values. At decrease pH, the carboxyl group is protonated, inflicting the hydrogel to shrink, whereas at larger pH, it’s deprotonated and ionized, resulting in repulsion between polymers and subsequent launch of insulin-loaded polymers. Research of the in vivo efficacy demonstrated induction of hypoglycemic results within the diabetic mice at 2 h after oral administration, which lasted for at the very least 6 h. The relative bioavailability of insulin was ~ 4.43%. When it comes to in vivo hepatotoxicity, though the degrees of the liver enzymes, particularly alanine aminotransferase, aspartate aminotransferase, and lactate dehydrogenase elevated as in comparison with these noticed within the management, they weren’t altered compared to the reference values. There was no nephrotoxicity as effectively, as serum and urine creatinine and urine microprotein ranges had been all inside the reference ranges, regardless of a big improve, as in comparison with that noticed within the 0.9% saline management group [54].
β-cyclodextrin is a cone-shaped heptasaccharide with a hydrophobic head and hydrophilic tail that may enhance the solubility of chitosan in water. Yang, et al. [55] studied the polymeric hydrogel composed of carboxymethyl chitosan (CMC) grafted with carboxymethyl β-cyclodextrin (CMCD) (CMCD-g-CMC) and located that 92% of insulin was retained within the CMCD-g-CMC hydrogels after 2 h of incubation beneath simulated gastric circumstances, and the share launched elevated to 55% at pH 6.8 and 70% when the pH was additional elevated to 7.4. Upon testing of the efficacy of this polysaccharide-based hydrogel in vivo, a maximal discount of blood glucose ranges was noticed at 6 h after oral administration, which lasted for ~ 12 h, as in comparison with that noticed upon SC injection, which peaked at 2 h, indicating a chronic impact of the hydrogel, which is right to attenuate the incidence of hypoglycemic episodes in diabetic sufferers. The viability of Caco-2 cells after 24 h of incubation with CMCD-g-CMC hydrogel microparticles with concentrations starting from 12.5 to 1600 μg/mL was > 96%, and thus, this hydrogel is secure to be used in oral drug supply [55]. These promising outcomes led to a long-term follow-up examine of 4 weeks with as soon as every day dosing in diabetic rats, which additional confirmed that insulin-loaded CMCD-g-CMC hydrogels had been efficient in treating the signs of polyphagia, polydipsia, polyuria, and weight reduction, in addition to in enhancing fasting blood glucose ranges and oral glucose tolerance check outcomes [56].
Apart from direct encapsulation of insulin, the hydrogel will also be used as a part of the drug supply system, along with particles loaded with insulin, as seen within the insulin-loaded sodium dodecyl sulfate and β-cyclodextrin (SDS/β-CD) vesicles-chitosan hydrogel. The cationic insulin was entrapped both on the floor of the SDS/β-CD bilayer of the vesicles, inside their cavity, or each, the place the vesicles had been embedded into the CS hydrogel crosslinked with β-glycerol phosphate, to enhance the soundness. An in vitro launch examine revealed that the loaded insulin might be retained within the system at pH 2.5, whereas speedy launch was noticed on the pH ranges of 6.8 and seven.4, with or with out enzymes added to the medium, and the share of residual insulin after incubation within the simulated intestinal fluid was considerably larger within the drug-in-vesicle-in-gel system than within the insulin-loaded hydrogel with out using vesicles [57].
Just like chitosan and PMAA, polyacrylic acid (PAA) additionally comprises a carboxyl group; this equips it with a pH-sensitive swelling property, thus making it an acceptable polymer for incorporation into the hydrogel community [58]. PAA was grafted onto bacterial cellulose (BC) by way of electron beam radiation, to utilize the robustness of BC in supporting PAA-based hydrogels which can be mechanically weak and biodegradable. As anticipated, the bacterial cellulose-g-poly(acrylic acid) [BC-g-P(AA)] hydrogel was pH-responsive, as the quantity of insulin launched within the first 2 h of incubation within the simulated gastric fluid was < 10%, however that within the simulated intestinal fluid was 77%–89% in 5–6 h, relying on the ratio of the hydrogel parts. The BC-g-P(AA) hydrogel additionally reversibly lowered the transepithelial electrical resistance check values, with the impact being higher throughout the Caco-2 monolayer than throughout the Caco-2/HT29-MTX monolayer, probably as a consequence of mucus. This was additionally mirrored by the truth that the permeability of insulin elevated by 3.5- to five.9-fold, as in comparison with that of the management, which was an insulin resolution. In an in vivo examine in diabetic rats, a big hypoglycemic impact was noticed, with as much as a 42%–49% discount in blood glucose ranges at 4–5 h for various compositions, versus the negligible impact of oral insulin. The relative bioavailability ranged from 6.98% to 7.45%, in distinction to that of the oral insulin resolution (0.64%). The viability of V-79, Caco-2, and HT29-MTX cells remained above 90% for all concentrations of the ready hydrogels, though there was a downward pattern because the focus elevated. Histological examination revealed no pathological adjustments within the intestinal or abdomen tissues [58].
Carboxymethyl cellulose is one other kind of cellulose grafted with PAA [59]. Acrylate-grafted carboxymethyl cellulose (CMC-g-AA) additionally confirmed a pH-selective insulin launch profile, with lower than 10% launched after 2 h at pH 1.2, and 80.8%–95.8% launched after 6 h at pH 6.8. The share decreased with a rise within the proportion of CMC content material and a lower within the AA content material, because it was speculated {that a} lower within the proportion of AA may cut back the swelling of the hydrogel, leading to a decrease diffusion charge. In vivo research in diabetic rats confirmed a 27.6% lower in fasting blood glucose ranges at 6 h after intragastric administration of the hydrogel loaded with 60 IU/kg insulin, which remained considerably decrease than that of the oral insulin resolution at 8 h. The relative pharmacological availability was 6.35% in comparison with that of the oral insulin resolution, which was < 0.5%. MTT assay carried out on CMC-g-AA hydrogels with completely different concentrations as much as 1000 mg/L demonstrated a cell viability of > 90% [59].
The preclinical research associated to hydrogel formulations are summarized in Desk 4.
Strong oral dosage kind
NN1952 and insulin 338 (I-338) are two oral insulin formulations that use completely different GI permeation enhancement applied sciences (GIPETs) developed by Merrion Prescription drugs. NN1952 consists of fast-acting insulin 106, which is modified from human insulin by substituting Tyr-α14 and Phe-β25 with glutamine and histidine, respectively, and omitting Thr-β30, to cut back the susceptibility of insulin to gastric acid denaturation and proteolytic enzyme motion [60]. Insulin 106 is tableted into GIPET-I, an enteric-coated pill of insulin and medium-chain fatty acids in particular ratios [61], to extend the oral bioavailability of the in any other case poorly permeable insulin. I-338 is a long-acting basal insulin analog with sodium caprate because the absorption enhancer that modulates the tight junctions between epithelial cells and fluidity of the cell membranes [60, 62]. Just like N1952, I-338 has been tableted into GIPET-I, with the insulin in it acylated by linking it to an 18-carbon fatty acid Desk 5.
Enteric-coated oral insulin capsules (ORMD-0801) have been used to deal with each type-1 and type-2 diabetes mellitus. The capsule additionally comprises a soybean trypsin inhibitor, disodium ethylene-diamine tetraacetic acid, which improves absorption throughout the intestinal epithelium; Aerosil 200 as a stabilizer; and Tween 80 as a surfactant [63]. Preclinical research on ORMD-0801 have been performed in pigs and canine. Pig fashions with intestinal entry that bypasses gastric digestion had been used, and ORMD-0801 lowered the AUC of blood glucose ranges by 7.0%–7.5% in them [64]. ORMD-0801 has additionally been used with and with out glucagon-like peptide 1 to manage postprandial glucose. Upon administering enteric-coated capsules immediately into the duodenum of the pigs, ORMD-0801 suppressed blood glucose ranges for a portion of the monitoring interval, in distinction to the rise noticed within the group that acquired the placebo [65].
The pharmacokinetic and pharmacodynamic (PK/PD) profiles upon oral administration of ORMD-0801 had been investigated in non-diabetic, wholesome beagle canines and in comparison with these obtained upon with duodenal administration and remedy with insulin via the SC route. The utmost exogenous insulin focus was highest for oral ORMD-0801. The Tmax for oral ORMD-0801 was 0.75 h, whereas these for duodenally administered ORMD-0801 and SC insulin had been 0.5 and 0.38 h, respectively. The imply AUC of insulin upon oral administration was much like that obtained for duodenal administration of ORMD-0801, which was higher than that of SC insulin. The imply relative bioavailability was 5.41%, and the onset of motion was 15 min after administration [66].
To beat one of many biggest limitations to oral insulin absorption, that’s, GI permeability, Eligen® expertise has been employed, the place insulin is non-covalently complexed with a permeation enhancer akin to monosodium N-(4-chlorosalicyloyl)-4-aminobutyrate (4-CNAB), which permits this macromolecule to be transported throughout the epithelial cells with out inflicting histological damages or adjustments such because the opening of tight junctions [67,68,69]. Capsulin is one other formulation that may enhance the permeation of insulin via enteric coating and incorporation of insulin, fragrant alcohols, and a dissolution support [70]. This formulation permits speedy dissolution upon contact with the intestinal epithelial lining.
Modified insulin
Two distinct merchandise have been created by altering the unbound amino acid on the Lys-β29 residue of artificial human insulin. The addition of a small hydrophilic oligomer to the construction of insulin enhances its solubility and stability. That is because of the hydrophilic nature of the extra oligomer and the steric hindrance it creates, which prevents the enzymes within the GI tract from binding to insulin.
The oral pill type of insulin analog IN-105 was chemically modified by attaching a methoxy triethylene glycol propionyl unit to the Lys-β29 residue of recombinant human insulin utilizing a non-hydrolyzable amide bond [71, 72]. The solubility of the formulation was enhanced by including PEG, which readily dissolves in water. Alternatively, hexyl insulin monoconjugate 2 (HIM-2), which includes the formation of a covalent connection between the Lys-29 of insulin and an amphiphilic oligomer, was collaboratively developed by Nobex Company and Biocon [73]. This stable oral dose, enclosed in a tough gelatin capsule, can inhibit the breakdown of proteins and enhance their absorption within the intestines.