Characterization of MWCNTs-PEI-AuNPs nanohybrids
Transmission electron microscopy (TEM) was utilized to characterize the morphology of the MWCNTs-PEI-AuNPs nanohybrids (Synthesized diagram proven in Fig. S1A). As introduced in Fig. S1B and S1C, the diameter of the MWCNTs (round 14 nm) elevated after coating with PEI (round 18 nm). Excessive-resolution transmission electron microscopy (HRTEM) pictures (Fig. S1D) clearly exhibited the interface between the sidewalls of the MWCNTs and the PEI. After HAuCl4 remedy, darkish particles had been noticed connected to the nanotubes (Fig. S1E). HRTEM picture in Fig. S1F indicated that AuNPs with a diameter of roughly 8 nm had been uniformly dispersed on the sidewalls of the nanotubes, forming MWCNTs-PEI-AuNPs. The UV-vis spectra (Fig. S1G) exhibited an absorption peak close to 520 nm after loading with AuNPs, which may be attributed to the absorption of the AuNPs [22]. These outcomes confirmed the profitable development of MWCNTs-PEI-AuNPs nanohybrids. To investigate the electrochemical efficiency of the MWCNTs-PEI-AuNPs, Cyclic voltammetry (CV) responses of various modified electrodes had been examined. Fig. S2 illustrated that, in comparison with the naked GCE, the height currents sequentially elevated after modifying with MWCNTs and MWCNTs-PEI-AuNPs. This indicated sooner and extra wonderful electron switch within the nanohybrids, suggesting their wonderful electrical conductivity. Due to this fact, electrodes functionalized with MWCNTs-PEI-AuNPs may considerably speed up electron switch and act as anchoring substrates for biomolecules, reminiscent of aptamers, thereby enhancing the efficiency of electrochemical biosensors.
Solubility, stability, formal potential and kinetic parameters of candidate mediators
The electrochemical aptasensor was constructed based mostly on HRP with H2O2 and a redox mediator, with HQ being the traditional mediator for this response system. To attain larger analytical sensitivity and specificity, we sought a novel and environment friendly mediator for the next HER2 aptasensor development. An efficient redox mediator ought to exhibit good solubility and stability, excessive electron switch exercise, and an acceptable formal potential relative to Ag/AgCl [24]. To pick the optimum mediator, we comparatively evaluated the solubility, mild stability, formal potential (vs. Ag/AgCl), and obvious fee fixed (okayapp) of the phenolic compounds, CAT, p-AP, APAP, and 2AP, as compared with HQ. The fundamental properties of those 5 compounds had been summarized in Desk 1.
HQ, CAT, and APAP exhibited larger solubility and dissolved by oscillation, whereas 2AP required sonication, and p-AP, with even decrease solubility, dissolved solely after extended sonication (Desk 1). Due to this fact, the solubility of HQ, CAT, APAP, and 2AP may be thought of ample. Ideally, the mediator must be extremely proof against mild, so we dissolved the 5 compounds in PBS and left them at room temperature with out mild safety for 1 h. Fig. S3 demonstrated that APAP didn’t change shade, demonstrating higher mild stability than the opposite compounds. 2AP remained largely undiscolored, indicating primary stability to mild, whereas the sunshine stability of HQ, CAT, and p-AP was poor.
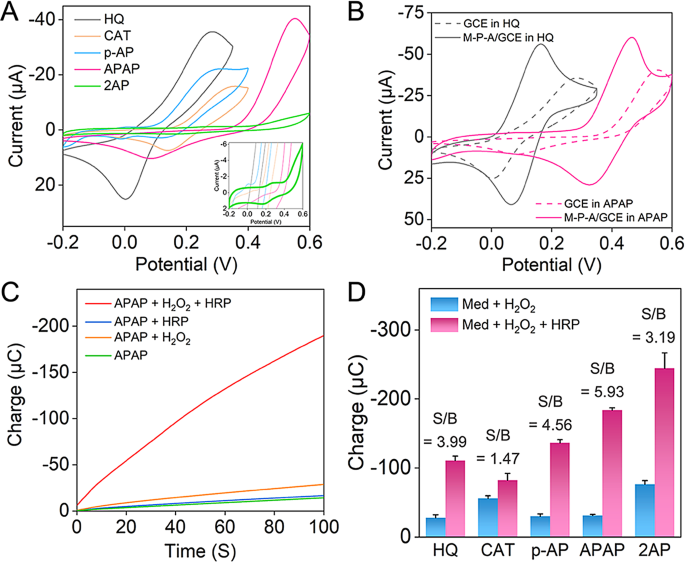
To optimize electron switch charges and sign amplification on this response system, the mediator’s formal potential must be fastidiously tuned. Ideally, it must be larger than the traditional mediator HQ’s formal potential, but decrease than that of HRP (0.7 V) [25]. We recorded CV curves (Fig. 1A) of the 5 mediators in PBS with a GCE electrode to review their electrochemical behaviors, and Fig. S4 demonstrated the electron switch mechanisms of the 5 compounds. Desk 1 listed the approximate formal potentials derived from the height potentials within the CV curves. In PBS, the formal potentials had been as follows: HQ (0.14 V), CAT (0.25 V), p-AP (0.21 V), APAP (0.32 V), and 2AP (0.23 V). Particularly, APAP possessed probably the most appropriate formal potential for our electrochemical aptasensor. Upon modification of the GCE with MWCNTs-PEI-AuNPs, a major enhancement in redox peak currents and a narrowing of peak spacing had been noticed for the mediators (Fig. 1B and S5). This statement highlighted the electrocatalytic exercise of MWCNTs-PEI-AuNPs in mediating the redox response of the mediator (within the absence of an electrochemical response). This catalytic exercise facilitated cost switch and amplified present indicators, per the findings introduced in Fig. S2. Importantly, even with the electrode modification, APAP maintained the very best formal potential among the many 5 mediators. As depicted in Fig. 1B, the formal potential of APAP remained considerably larger than that of the traditional mediator HQ (roughly 0.18 V larger).
Analysis of the candidate redox mediators. (A) CV responses obtained utilizing GCE electrodes in 0.1 M PBS (pH 7.4) containing 2 mM totally different mediators. Inset: CV response of 2AP. (B) CV responses obtained utilizing MWCNTs-PEI-AuNPs modified- or naked GCE electrodes in 0.1 M PBS (pH 7.4) containing 2 mM HQ or APAP. (C) Chronocoulometric curves obtained utilizing GCE electrodes at 0.0 V in 0.1 M PBS (pH 7.4) containing 2 mM APAP, PBS containing 2 mM APAP and three mM H2O2, PBS containing 2 mM APAP and 40 µg mL-1 HRP, and PBS containing 2 mM APAP, 3 mM H2O2, and 40 µg mL-1 HRP. (D) Cost values at 100 s from chronocoulograms utilizing GCE electrodes in 0.1 M PBS (pH 7.4) containing 2 mM mediators and three mM H2O2, and PBS containing 2 mM mediators, 3 mM H2O2, and 40 µg mL-1 HRP
To confirm the viability of the candidate compounds as mediators within the electrochemical-enzymatic redox cycle, naked electrodes had been subjected to chronocoulometry after incubation in numerous options. As depicted in Fig. 1C, the cost values exhibited a marginal enhance when both H2O2 or HRP was added to the APAP resolution, implying the direct response between APAP and H2O2 or HRP was sluggish. Nonetheless, the chronocoulogram exhibited a considerably steeper slope when each H2O2 and HRP had been launched to the APAP resolution. This indicated the incidence of a speedy and extremely environment friendly electrochemical-enzymatic redox cycle, facilitating electron switch. This statement aligned with the phenomenon noticed in HQ (Fig. S6A). Likewise, related outcomes had been obtained for CAT, p-AP, and 2AP (Fig. S6B–D), suggesting their feasibility as candidate mediators within the electrochemical-enzymatic redox cycle of HRP.
Then, we evaluated the electron switch kinetics of the 5 compounds. This evaluation utilized okayapp calculated using Eq. 5 for the HRP-mediated redox system [26].
$${I}_{lim}=2FA{C}_{M}sqrt{D{okay}_{app}{C}_{E}}$$
(5)
the place Ilim, F, A, CM, D, and CE characterize the background-corrected restrict present, Faradaic fixed, electrode floor space, mediator focus, mediator diffusion coefficient, and HRP focus, respectively. Values for Ilim had been obtained from the chronoamperograms depicted in Fig. S7. Diffusion coefficients for every of the 5 compounds had been decided by measuring restrict currents. This measurement employed an ultramicroelectrode via linear scanning voltammetry (Fig. S8) and was calculated based on Eq. 6 [26].
the place n is the variety of transferred electrons, C is the mediator focus, and r is the radius of the ultramicroelectrode. Calculated diffusion coefficients, in addition to okayapp, for the 5 compounds in PBS, had been introduced in Desk 1. The okayapp values for each APAP and 2AP had been related, at roughly 2.8 × 105 M− 1 s− 1. These values had been larger than these of the opposite compounds, particularly HQ, revealing their potential to be utilized as extremely environment friendly electron mediators for enzymatic reactions.
To successfully make the most of the mediator within the following electrochemical evaluation, reaching a low detection restrict and excessive selectivity required minimizing the electrochemical sign within the absence of HRP, whereas maximizing sign enhancement upon HRP introduction. Due to this fact, we evaluated the signal-to-background (S/B) ratios of the totally different mediators by measuring their expenses at 100 s. Among the many 5 mediators evaluated, APAP demonstrated the very best S/B ratio (Fig. 1D), suggesting that APAP was a superior mediator for delicate and particular electrochemical evaluation in comparison with the traditional mediator, HQ. These outcomes collectively pointed to APAP because the optimum redox mediator for this electrochemical-enzymatic response system, resulting in its deployment within the following electrochemical aptasensor research.
Design, synthesis and characterization of HRP-Ab-AuNPs@COF composites
AuNPs@COF composites had been synthesized via a facile solution-phase technique using TAPB and DMTP as precursors. The spherical COF, performing as a development template for the AuNPs, was ready via an aldimine condensation response. Then, antibodies had been immobilized onto the composite through Au-NH2 bonding, whereas HRP was built-in within the COF pores via host-guest interactions (Scheme 1 A) [27]. TEM was employed to research the morphology of the synthesized supplies. As depicted in Fig. 2A, the COF exhibited a uniform spherical morphology with a tough floor. Following the in-situ development of AuNPs on the COF, uniformly dispersed darkish particles had been noticed on its floor, with no proof of aggregation or free AuNPs (Fig. 2B). This statement implicated that the COF construction facilitated the expansion of AuNPs. Vitality dispersive X-ray spectroscopy (EDS) elemental mapping confirmed the presence of C, N, O, and Au parts, all important for the profitable synthesis of AuNPs@COF. As well as, the mapping evaluation verified the uniform distribution of Au on the COF floor (Fig. 2C-I).
The Fourier remodel infrared spectra (FT-IR) had been introduced in Fig. 3A. The absorption peaks at 3430, 3346, and 3208 cm− 1 for TAPB arose from N-H vibrations [18]. The absorption peak at 2759 cm− 1 for DMTP was attributed to C-H vibrations within the aldehyde group, whereas the height at 1676 cm− 1 corresponded to C = O bonding. The synthesized COF exhibited attribute peaks at 1618, 1465, and 1288 cm− 1, assigned to C = N, CH3, and C-O, respectively [28]. These had been accompanied by a weakening of the N-H stretching vibration peaks of TAPB and the C = O stretching vibration peak of DMTP, providing robust proof for profitable aldimine condensation. Upon the introduction of AuNPs to the COF, the positions of the attribute absorption peaks remained largely unchanged, indicating that the AuNPs didn’t considerably have an effect on the COF construction. Nonetheless, a slight shift was noticed for the height at 3372 cm− 1 to 3366 cm− 1, doubtlessly resulting from interactions between [AuCl4]− and NH3+. The XRD sample of AuNPs@COF, depicted in Fig. 3B, indicated extra diffraction peaks at 38.08°, 44.28°, and 64.47° in comparison with COF. These peaks corresponded to the (111), (200), and (220) reflective surfaces of Au, respectively [7]. X-ray photoelectron spectroscopy (XPS) (Fig. S9) confirmed the presence of C, N, O, and Au parts in AuNPs@COF, per the EDS outcomes (Fig. 2). Within the N 1s spectrum (Fig. 3C), two fitted peaks had been noticed, akin to C = N and residual -NH2, additional supporting the profitable synthesis of COF. The Au 4f spectrum displayed two peaks at 87.38 and 83.68 eV, representing the Au(0) 4f5/2 and 4f7/2, respectively (Fig. 3D) [29]. Particularly, the binding power of N 1s in AuNPs@COF was decrease in comparison with COF, suggesting a robust interplay between AuNPs and -NH2. Contemplating the outcomes holistically, the profitable synthesis of AuNPs@COF could possibly be confirmed.
Synthesis and characterization of AuNPs@COF. (A) FT-IR spectra of TAPB, DMTP, COF, and AuNPs@COF. (B) XRD patterns of COF and AuNPs@COF. (C) XPS spectra of N 1s in COF and AuNPs@COF. (D) XPS spectra of Au 4f in AuNPs@COF. (E) Nitrogen adsorption-desorption isotherms, (F) pore measurement distribution, and (G) TGA curves of COF and AuNPs@COF
Nitrogen suction-desorption experiments had been employed to research the pore traits of AuNPs@COF. As depicted in Fig. 3E, COF displayed kind IV isotherms at 77 Ok, indicating mesoporous properties. The Brunauer-Emmett-Teller (BET) particular floor space of COF decreased from 246.1 m2 g− 1 to 35.6 m2 g− 1 after the introduction of AuNPs, whereas the mesoporous construction was retained. NLDFT evaluation (Fig. 3F) indicated that the pore volumes of COF earlier than and after loading with AuNPs had been 0.425 cm3 g− 1 and 0.144 cm3 g− 1, respectively. This discount in BET particular floor space and pore quantity advised that the AuNPs had been possible positioned both inside or on the floor of the COF. As well as, thermogravimetric evaluation (TGA) indicated good thermal stability for each COF and AuNPs@COF as much as 400 °C (Fig. 3G). The abundance of open mesopores within the synthesized AuNPs@COF not solely facilitated the loading of antibodies and enzymes but additionally enhanced cost switch and molecular diffusion through the catalytic course of.
Lastly, to confirm the profitable synthesis of HRP-Ab-AuNPs@COF, we employed CV to appraise electrodes modified with numerous parts. These electrodes had been immersed in PBS resolution containing each APAP and H2O2. As proven in Fig. S10, the naked electrode, together with electrodes modified with AuNPs@COF and Ab-AuNPs@COF, exhibited solely capacitive currents. In distinction, the electrode modified with HRP-Ab-AuNPs@COF displayed vital cathodic currents. This statement pointed to the presence of enzymatic redox reactions, facilitating electron switch, and thus confirming the profitable synthesis of HRP-Ab-AuNPs@COF.
Catalytic and electrochemical efficiency of HRP-Ab-AuNPs@COF
Leveraging the catalytic properties of HRP, the interplay with H2O2 results in the formation of the [HRP + H2O2] advanced. This complexation, accordingly, facilitates the oxidation of a spread of hydrogen donors, with TMB representing an acceptable candidate, considerably exhibiting vital absorption at 652 nm upon oxidation (ox-TMB) [23]. To guage the catalytic exercise of each free HRP and HRP-Ab-AuNPs@COF within the presence of H2O2, the TMB colorimetric technique was employed. It must be famous that immobilized HRP portions had been normalized to equal free HRP quantities using the immobilization effectivity (see extra dialogue in Desk S1). Our preliminary analyses centered on steady-state catalytic kinetics using a mix of free or immobilized HRP with TMB, whereas various the concentrations of H2O2 (Fig. S11). The V0 of HRP, decided at totally different H2O2 concentrations, was then calculated via the Beer-Lambert Regulation, facilitating a complete analysis of catalytic exercise. Michaelis-Menten curves had been plotted, representing the connection between H2O2 focus and corresponding V0 values (Fig. 4A and C). As well as, Lineweaver-Burk plots had been constructed using linear double inverse transformations (Fig. 4B and D). The kinetic parameters Vmax and Okm had been then calculated to supply a quantitative evaluation of catalytic capability. Particularly, our outcomes demonstrated that the Okm worth (2.69 mM) of HRP-Ab-AuNPs@COF was decrease in contrast with that of free HRP, indicating a major enhance in catalytic effectivity. This was primarily attributed to the mesoporous construction and domain-limiting impact of COF. These properties allowed for coupling between inside diffusion and the catalytic response, successfully eliminating diffusion limitations. Accordingly, this elevated the focus of native catalytic websites and mediators, lastly enhancing substrate mass switch effectivity. In the meantime, the host-guest interplay between HRP and COF scaffold resulted in a partial discount within the most response fee, as evidenced by the decrease Vmax by the immobilized enzyme. Nonetheless, for sure immobilized enzymes, a slight enhance within the preliminary response fee was noticed, which we hypothesized to be a consequence of the substrate channeling impact, i.e., the general catalytic turnover fee of the immobilized enzyme was larger than that of the randomly distributed free enzyme in resolution [30]. Moreover, the synergistic impact launched by the Au particles connected within the COF pores, doubtlessly exhibiting enzyme-mimicking exercise, could have acted as a synergistic booster, thereby enhancing the preliminary response fee to a sure extent [31, 32].
Comparative evaluation of the catalytic and electrochemical efficiency between free HRP and HRP-Ab-AuNPs@COF. Michaelis-Menten becoming curves of (A) free HRP and (C) HRP-Ab-AuNPs@COF. Lineweaver-Burk plots obtained from Michaelis-Menten becoming curves of (B) free HRP and (D) HRP-Ab-AuNPs@COF. (E) Stability take a look at of free HRP and HRP-Ab-AuNPs@COF beneath totally different storage situations. (F) CV responses and (G) histogram for the present values of free HRP and HRP-Ab-AuNPs@COF in 0.1 M PBS (pH 7.4) containing 2 mM APAP and three mM H2O2. *p < 0.05 by unpaired t take a look at
The catalytic stability of HRP-Ab-AuNPs@COF was evaluated using a colorimetric TMB assay. As depicted in Fig. 4E, HRP-Ab-AuNPs@COF retained 97.5% of its catalytic exercise after 15 days of storage at 4 °C, marginally surpassing free HRP (94.1%). Moreover, HRP-Ab-AuNPs@COF exhibited exceptional stability at 37 °C, retaining 91.9% of its catalytic exercise after 15 days. In distinction, free HRP displayed a major decline in exercise beneath an identical situations, with its exercise dropping under 90% after solely 7 days (in comparison with 95.1% for HRP-Ab-AuNPs@COF) and declining to a mere 81.8% after 15 days. This superior catalytic stability of HRP-Ab-AuNPs@COF could possibly be attributed to the protecting encapsulation and anchoring interactions supplied by the COF construction.
To confirm the benefits of HRP-Ab-AuNPs@COF, we evaluated the electrochemical properties of each the novel construction and free HRP. Determine 4F demonstrated the CV curves of each free HRP and HRP-Ab-AuNPs@COF in PBS containing APAP and H2O2. Particularly, the present of HRP-Ab-AuNPs@COF was 53.3% larger than that of free HRP (Fig. 4G). This confirmed that the HRP-Ab-AuNPs@COF construction exhibited considerably stronger catalytic efficiency and sign amplification. These outcomes advised that HRP-Ab-AuNPs@COF could possibly be utilized as an environment friendly catalyst for future research.
Fabrication and optimization of the aptasensor
An electrochemical aptasensor was developed for delicate and particular detection of HER2. The sensor’s electrode floor was modified with a layer of MWCNTs-PEI-AuNPs, which enhanced electron switch and supplied a scaffold for aptamer immobilization. Upon introduction of HER2, the aptamer selectively certain to the goal, which was then captured by the HER2 Ab conjugated to COF-based composites (Fig. 5A). This complexation then facilitated an enzymatic response: HRP immobilized on the COF catalyzed the oxidation of APAP within the presence of H2O2. The oxidized APAP was then diminished electrochemically, creating an electrochemical-enzymatic redox cycle that amplified the sign for detection (Scheme 1 C). Particularly, the binding websites of the aptamer and the antibody in opposition to HER2 had been evaluated using floor plasmon resonance (SPR) assay, which demonstrated that they two focused totally different HER2 binding websites and had been appropriate for the development of the aptasensor (see Fig. S12 for extra dialogue).
Institution and analytical efficiency of the aptasensor. (A) Schematic description of the HER2 aptasensor meeting. (M-P-A refers to MWCNTs-PEI-AuNPs, H-A-A@C refers to HRP-Ab-AuNPs@COF). (B) CV responses of (a) naked GCE, (b) MWCNTs-PEI-AuNPs/GCE, (c) HER2 aptamer/MWCNTs-PEI-AuNPs/GCE, (d) MCH/HER2 aptamer/MWCNTs-PEI-AuNPs/GCE, (e) HER2/MCH/HER2 aptamer/MWCNTs-PEI-AuNPs/GCE, (f) HRP-Ab-AuNPs@COF/HER2/MCH/HER2 aptamer/MWCNTs-PEI-AuNPs/GCE in 0.1 M PBS (pH 7.4) containing 2 mM Ok3Fe(CN)6. (C) Chronocoulometric curves and (D) calibration plot of the introduced aptasensor with totally different concentrations of HER2 in 0.1 M PBS (pH 7.4) containing 2 mM APAP and three mM H2O2. (E) Stability of the as-fabricated aptasensor for 1 ng mL− 1 HER2 after a storage of two, 7, and 15 days. (F) Cost values of the as-fabricated aptasensor for HER2 and interfering substances with a focus of 1 ng mL− 1. ***p < 0.001 and ns, not vital by one-way ANOVA. (G) Comparability of cost values of the APAP-mediated system and the HQ-mediated system for PD-L1, CEA, CA15-3, and HER2
CV was employed to review the stepwise meeting of the aptasensor. As depicted in Fig. 5B, the naked GCE exhibited a well-defined [Fe(CN)6]3− redox peak with a peak-to-peak separation of lower than 80 mV (curve a), indicating facile electron switch kinetics. The incorporation of MWCNTs-PEI-AuNPs, identified for his or her wonderful electron switch properties, led to a major enhance in peak present on the functionalized electrode (curve b). The successive change with the aptamer, MCH, HER2, and HRP-Ab-AuNPs@COF resulted in a progressive lower in peak currents. This statement was attributed to the hindered penetration of the redox probe and impeded electron switch attributable to the presence of non-electroactive proteins and molecules. These findings confirmed the profitable meeting of the aptasensor.
To attain optimum sign output from the aptasensor, we performed a sequence of optimization experiments, using chronocoulometry to observe the consequences of various development situations. Recognizing the direct impact of aptamer focus on detection effectiveness, we first optimized this parameter. As illustrated in Fig. S13A, absolutely the cost worth recorded at 100 s exhibited an growing development with growing aptamer concentrations, lastly reaching a plateau at 1 µM. This saturation level indicated full binding of the aptamer to the MWCNTs-PEI-AuNPs substrate, establishing 1 µM because the optimum aptamer focus for the next experiments. Then, we explored the numerous impact of incubation temperature on detection sensitivity. We chosen two consultant incubation temperatures, 4 °C and 37 °C, for this evaluation. As depicted in Fig. S13B, each HER2 and HRP-AuNPs@COF yielded most absolute cost values when incubated at 4 °C. Due to this fact, we designated 4 °C because the optimum incubation temperature for each HER2 and HRP-Ab-AuNPs@COF. Lastly, we evaluated the vital position of incubation time in optimizing the detection sign. Our findings demonstrated that an incubation time of 0.5 h constantly yielded the very best absolute cost values for the aptamer, HER2, and HRP-Ab-AuNPs@COF (Fig. S13C). Primarily based on these outcomes, we established 0.5 h because the optimum incubation time for the aptamer, HER2, and HRP-Ab-AuNPs@COF.
Analytical efficiency of the HER2 aptasensor
Below optimum experimental situations, the developed aptasensor, using chronocoulometry with APAP because the redox mediator, efficiently detected HER2. As anticipated, absolutely the cost exhibited a optimistic correlation with growing HER2 concentrations (Fig. 5C). A linear relationship was noticed between absolutely the cost at 100 s and the logarithm of HER2 focus over a spread of 0.5 pg mL− 1 to 100 ng mL− 1 (Fig. 5D). This relationship was outlined by the linear regression equation Q (µC) = − 47.766 lg [HER2 (ng mL− 1)] − 208.13, with a correlation coefficient of 0.993. As well as, the restrict of detection (LOD) was decided to be 0.418 pg mL− 1 (S/N = 3). This aptasensor demonstrated superior analytical efficiency in comparison with quite a few beforehand reported biosensors designed for HER2 detection (Desk S2). To guage stability, the constructed aptasensors had been saved at 4 °C for two, 7, and 15 days. Particularly, the electrochemical sign responses remained at 97.638%, 94.025%, and 93.150% of the preliminary response, respectively (Fig. 5E), indicating passable stability. The coefficient of variation (RSD) of cost, representing the dedication of 1 ng mL− 1 of HER2 using 5 independently fabricated aptasensors, was 3.275% (Fig. S14). This outcome confirmed the nice reproducibility of the aptasensing platform.
To find out the selectivity of the newly developed electrochemical analytical technique, 1 ng mL− 1 PD-L1, CEA, CA15-3, and mixtures of those potential interferents with HER2 had been individually evaluated alongside HER2 beneath an identical experimental situations. Determine 5F illustrated that absolutely the expenses of the interferents at 100 s had been considerably decrease than that of HER2. Nonetheless, absolutely the expenses of the combination had been just like that of HER2, indicating wonderful aptasensor selectivity. This was possible attributable to the environment friendly enzymatic response and the excessive affinity and particular recognition of HER2 by the aptamer or antibody. For comparability, an aptasensor using HQ because the electron mediator was additionally constructed (schematic demonstrated in Fig. S15). As depicted in Fig. 5G, the APAP-mediated aptasensor detected solely 59–73% of the interfering agent indicators in comparison with the HQ-mediated counterpart. This outcome clearly demonstrated that APAP exhibited superior selectivity and immunity to interferents in comparison with HQ, probably the most generally utilized mediator. To guage the accuracy of this technique, restoration experiments had been carried out using the developed aptasensor for numerous concentrations of HER2 in wholesome human serum. The analytical recoveries ranged from 91.104 to 102.925% with an RSD in 7.531% (Desk S3), confirming the aptasensor’s accuracy and reliability. The excessive sensing efficiency was attributed to the extremely environment friendly electron mediator APAP and the excessive catalytic efficiency of COF-immobilized HRP.
Medical utility of the HER2 aptasensor
To discover the scientific viability of a newly developed enzyme-enhanced electrochemical aptasensor, we utilized it to research HER2 ranges within the tradition supernatants of a number of cell traces, together with the human mammary epithelial line MCF-10 A and the human breast most cancers cell traces MDA-MB-231, MCF-7, and ZR-75-1. Our evaluation indicated that the breast most cancers cells possessed the next absolute cost in comparison with regular mammary epithelial cells (Fig. S16). This advised that breast most cancers cells could launch extra HER2 than their regular counterparts, thus illustrating the sensible utility of the aptasensor.
Subsequently, the aptasensor was employed to research serum samples from sufferers with benign and malignant breast illnesses. Affected person info was obtainable in Tables S4 and S5. As introduced in Fig. 6A, we measured the cost of scientific samples from wholesome people, sufferers with benign breast illness (BBD), and sufferers with breast most cancers (BC). By evaluation, HER2 ranges had been discovered to be considerably larger in BC sufferers in comparison with each BBD sufferers and wholesome people (Fig. 6B). Then, we in contrast HER2 ranges in BC sufferers at totally different scientific levels (Fig. 6C; affected person info was obtainable in Tables S6 and S7). We decided that samples from metastatic BC sufferers displayed considerably larger absolute cost than these from non-metastatic BC sufferers (Fig. 6D). Importantly, the various intensities of electrochemical indicators noticed in samples from BC sufferers had been largely per BC development, suggesting that HER2 could also be a worthwhile biomarker for the scientific prognosis of BC. Using receiver working attribute (ROC) curve evaluation, an optimum diagnostic cut-off worth of -220.90 µC for HER2 was established (Fig. 6E). The aptasensor demonstrated excessive diagnostic accuracy for breast most cancers (space beneath the curve, AUC = 0.928), with good accuracy (84.44%) and excessive optimistic (PPV) and unfavourable (NPV) predictive values (Desk-Fig. 6E, PPV = 90.91%, NPV = 78.26%). Particularly, the AUC worth and accuracy of HER2 detection using the developed aptasensor had been considerably larger than these achieved with standard biomarkers, reminiscent of CEA (AUC = 0.702, see Fig. S17A for particular info) and CA15-3 (AUC = 0.807, see Fig. S17B for particular info), as measured by scientific immunoassay evaluation. These outcomes indicated that, in comparison with the usage of classical biomarkers in customary strategies, this aptasensor extra successfully differentiated between BC sufferers and wholesome people, in addition to between BC sufferers at totally different scientific levels, considerably bettering the accuracy of breast most cancers prognosis. Thus, this high-performance aptasensor had robust potential for scientific utility.
Utility of the aptasensor in scientific serum samples. (A) HER2 ranges in scientific serum samples derived from wholesome people (n = 10), BBD sufferers (n = 10), and BC sufferers (n = 10) measured utilizing the aptasensor. (C) HER2 ranges in scientific serum samples derived from non-metastatic (n = 8) and metastatic (n = 7) BC sufferers measured utilizing the aptasensor. (B and D) Field plots mirrored the statistical outcomes of the decided HER2 ranges proven in (A and C), respectively. *p < 0.05, **p < 0.01, ***p < 0.001 by unpaired t take a look at. (E) ROC curve and diagnostic testing analysis elucidated the worth of this technique for breast most cancers prognosis