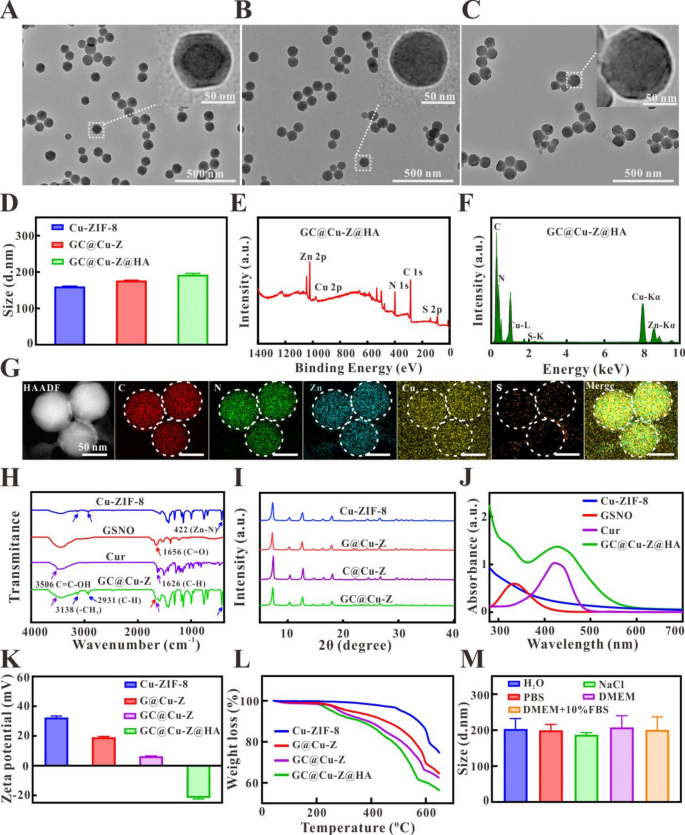
Synthesis and characterization of nanocarrier
ZIF-8 is broadly used because the drug service resulting from its traits of fantastic drug loading skill, pH-response and adjustable drug launch. Due to this fact, Cu-doped ZIF-8 (Cu-ZIF-8) was firstly ready in response to the literature [39]. The doped Cu have essential impact on the next therapy effectivity, the doping charge of Cu in ZIF-8 was optimized to realize most stage. The impact of on the Cu-ZIF-8 was explored by adjusting the content material of Cu. It was confirmed that the Transmission electron microscopy (TEM) photos of Cu-ZIF-8 ready with totally different Cu2+/2-MI molar ratios. The scale and dope charge of the crystals elevated with the rise of Cu2+/2-MI molar ratio. The Cu-ZIF-8 nanoparticle with measurement round 50 nm had been obtained when the feeding molar ratio of Cu2+/2-MI was 0.02, and the dope charge was about 3.8% by atomic absorption spectrophotometer (AAS) (Fig. S1A). Because the ratio elevated to 0.05, the scale additionally elevated to 80 nm, and dope charge raised to six.5% (Fig. S1B). Whereas the Cu2+ content material was additional elevated to a Cu2+/2-MI ratio of 0.2, the scale was elevated to 180 nm, and the dope charge was elevated to eight.6% (Fig. S1C). Comparable phenomenon was additionally noticed in earlier examine [40]. The elevated measurement of Cu-ZIF-8 NPs with growing Cu2+ quantities could be attributed the 2-MI produced numerous nuclei, which accelerated crystal nucleation, resulted in lengthen crystal development. Due to this fact, with a view to get hold of the Cu-ZIF-8 with the utmost doping, massive particular floor space and acceptable measurement, the feeding molar ratio of 0.05 for Cu2+/2-MI was chosen for subsequent experiments. TEM confirmed that Cu-ZIF-8 offered geometric dodecahedral nanostructure with uniform distribution, with a measurement of about 85 nm (Fig. 2A). After loading GSNO and Cur, the GC@Cu-Z nanoparticle was spherical and effectively dispersed, and the scale was roughly 90 nm (Fig. 2B), because of the loaded curcumin incorporates phenolic hydroxyl group, which might competitively coordinate with 2-MI, and the electronegativity of -OH containing lone pair electrons is stronger than that of -NH [41]. Since these two small molecule medicine had been concurrently encapsulated into Cu-ZIF-8, the impact of various ratio of service and drug on the nanocarrier was additionally explored. On this experiment, the quantity of GSNO and Cur was managed to be identical, when the ratio of service to drug was 8:1 and 4:1, TEM end result demonstrated that GC@Cu-Z exhibited apparent aggregation (Fig. S2), the morphology dramatically modified, and the hydrated particle measurement and polydispersity index (PDI) turned bigger. Whereas the ratio was 2:1 and 1:1, the morphology and construction of GC@Cu-Z remained intact, and the hydrated particle measurement and PDI had been considerably decreased. An analogous phenomenon was noticed when Cu-ZIF-8 nanocarrier was modified by HA (Fig. S3). Therefore, the ratio of two:1 was chosen to organize GC@Cu-Z@HA for complete consideration. The floor of the nanocarrier obtained on this case was tough, and the scale was barely elevated to about 100 nm with good dispersion (Fig. 2C). Dynamic gentle scattering (DLS) displayed that the scale of Cu-ZIF-8 was about 160.3 nm (Fig. 2D), which was bigger than that of TEM, as a result of the previous was hydrated particle measurement. After encapsulation with GSNO and Cur, the scale elevated to 176.5 nm, after which 204.8 nm after HA modification, which was in step with the results of TEM.
X-ray photoelectron spectroscopy (XPS) and power dispersive spectroscopy (EDS) confirmed that the nanocarrier was composed of C, N, Zn, Cu, and S parts (Fig. 2E, F), and the fundamental mapping evaluation additional confirmed that these parts had been uniformly distributed within the GC@Cu-Z@HA nanocarrier (Fig. 2G). It was noteworthy as a result of the service mesh contained Cu, ensuing within the distribution of Cu component in the whole subject of view. Besides, the distribution of copper within the nanocarrier confirmed a transparent boundary distinction in comparison with different areas, which indicated that the nanocarrier was efficiently ready. Within the meantime, the fourier rework infrared (FTIR) spectroscopy of the nanomaterial was additionally acquired (Fig. 2H). For Cu-ZIF-8, it was noticed that Zn-N stretching vibration peak at 422 cm− 1, and aircraft bending and stretching vibration peaks of imidazole ring at 500 ~ 1350 and 1350 ~ 1500 cm− 1, respectively; for GSNO, a stretching vibration peak of C = O emerged at 1656 cm− 1; for Cur, allylic alcohol and phenolic hydroxyl teams at 3506 and 1656 cm− 1 [39, 42]. The discovering revealed that GC@Cu-Z had all of the above traits peaks, proving the profitable synthesis of GC@Cu-Z. The X-ray diffraction (XRD) patterns of GSNO@Cu-ZIF-8 (G@Cu-Z), Cur@Cu-ZIF-8 (C@Cu-Z), GSNO/Cur@Cu-ZIF-8 (GC@Cu-Z) confirmed related diffraction peaks as Cu-ZIF-8 (Fig. 2I), indicating that crystal construction of GC@Cu-Z was not modified after drug loading. The UV-vis absorption spectra displayed that the attribute absorption peaks of GSNO (340 nm) and Cur (430 nm) had been all appeared in GC@Cu-Z@HA (Fig. 2J). Then, the answer washed with methanol, with the rise of washing occasions, the supernatant had nearly no shade and no UV absorption peak (Fig. S4). In line with the usual absorption curves of GSNO and Cur (Fig. S5), evaluating the mass of Cu-ZIF-8 earlier than and after loading drug, the encapsulation efficiencies of GSNO and Cur had been 47.5% and 49.2%. Moreover, the content material of Cu was 5.7%, as measured by atomic absorption spectroscopy (AAS). As well as, the Zeta potential exhibited that the floor potential of Cu-ZIF-8 was 32.4 mV (Fig. 2Ok), that of GC@Cu-Z decreased to six.3 mV after loading GSNO and Cur, whereas modification with HA, it modified to -21.7 mV, which was as a result of HA was negatively charged hydrophilic ligand and may very well be modified to the GC@Cu-Z floor by electrostatic power.
Thermogravimetric evaluation (TGA) confirmed that the thermal degradation charge elevated considerably with the rise of temperature (40 to 650 °C) within the following order, GC@Cu-Z@HA > GC@Cu-Z > G@Cu-Z > Cu-ZIF-8 (Fig. 2L). This could be resulting from the truth that GC@Cu-Z@HA was modified with HA polymer, which had a decrease thermal degradation temperature and degraded quickly after temperature rising. Then, the loaded small molecule drug was subsequently degraded, which additional demonstrated the encapsulation of GSNO and Cur, and the profitable preparation of GC@Cu-Z@HA nanocarrier. In addition to, the steadiness of nanocarrier was essential for its utility. Due to this fact, in vitro simulated stability experiment was additionally carried out on GC@Cu-Z@HA. It demonstrated that the nanocarrier had no important change of hydration particle and PDI after incubating with H2O, PBS, NaCl, Dulbecco’s modified Eagle’s medium (DMEM), DMEM + 10% fetal bovine serum (Fig. 2M, S6), indicating that the nanocarrier had good stability in several media. Then, the modifications of hydrated particle measurement, Zeta potential and PDI of the nanocarrier with time at 4, 25 and 37 °C had been additionally measured, it displayed that the scale and potential remained principally steady (Fig. S7), whereas PDI had negligible change in ultrapure water. It may very well be seen that the multifunctional nanocarrier possessed glorious stability in several temperature and media, which laid the stable basis for the biomedical utility.
Characterization of nanocarrier. TEM and magnification (inset) photos of Cu-ZIF-8 (A), GC@Cu-Z (B) and GC@Cu-Z@HA (C), and their hydrated particle measurement (D); XPS (E), EDS (F) and elemental mapping (G) of GC@Cu-Z@HA; FTIR spectrum of Cu-ZIF-8, GSNO, Cur and GC@Cu-Z (H); XRD evaluation of Cu-ZIF-8, G@Cu-Z, C@Cu-Z and GC@Cu-Z (I); UV-vis absorption spectrum of Cu-ZIF-8, GSNO, Cur and GC@Cu-Z@HA (J); Zeta potential (Ok) and TGA (L) of Cu-ZIF-8, G@Cu-Z, GC@Cu-Z and GC@Cu-Z@HA; hydrated particle measurement distribution of GC@Cu-Z@HA in several media (M)
In vitro degradation and catalytic property of the nanocarrier
Within the design of nanocarrier, ZIF-8 was a type of pH-sensitive MOF, which remained steady underneath impartial situation and degraded in acidic atmosphere. The tumor microenvironment was characterised by the weak acidity and excessive GSH stage, then GC@Cu-Z@HA would degrade within the tumor website, thus triggering the discharge of GSNO and Cur. Firstly, the biodegradation attribute of GC@Cu-Z@HA was investigated. Within the experiment, GC@Cu-Z@HA was immersed within the resolution of various pH with or with out GSH and detected at totally different time factors. TEM evaluation confirmed that the construction of GC@Cu-Z@HA remained utterly intact and dispersed uniformly even after 4 h incubation in impartial resolution (Fig. 3A); comparatively, extra apparent degradation was noticed underneath acidic situation with the extension of time, the morphology of the nanoparticle turned markedly irregular, and the hydrated particle measurement elevated considerably (Fig. 3B). In the meantime, the degradation of GC@Cu-Z@HA additionally occurred if GSH was added within the impartial resolution, the same phenomenon was noticed in earlier examine, because of the addition of acidic peptide GSH selling the degradation of GC@Cu-Z@HA (Fig. S8) [43]. As well as, Cu2+ might react with GSH to generate Cu+ and GSSG by redox response, and the coordination between Cu+ and 1,2-dimethylimidazole (2-MI) was totally different from Cu2+, which could additionally promote the degradation of GC@Cu-Z@HA. Due to this fact, it may very well be seen that GC@Cu-Z@HA degraded quicker in acidic resolution containing GSH than with out GSH. This recommended that GC@Cu-Z@HA may very well be used nearly as good nanocarrier for drug launch in response to tumor microenvironment.
GSH, as the main intracellular antioxidant, performed a central function in defending cell from numerous sort of oxidation, and extra GSH in tumor might scavenge reactive oxygen species (ROS) to mitigate oxidative stress and promote development, significantly lowering the efficacy of CDT. In an effort to examine the GSH responsive property of the nanocarrier, 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB) was adopted because the thiol chromogenic agent to analyze the GSH depletion by GC@Cu-Z@HA. The end result confirmed that with the extension of response time, the absorption peak of the system regularly decreased (Fig. 3C), implying that the content material of GSH decreased constantly. And the quantitative evaluation of the absorption at 412 nm demonstrated that the lack of GSH might attain 75.93% after 12 h (Fig. 3D), proving that the GC@Cu-Z@HA might certainly successfully lower the content material of GSH. Since Cu2+ reworked into Cu+ throughout the depletion of GSH, and Cu+ might act because the Fenton-like reagent to catalyze the manufacturing of hydroxyl radical (∙OH). Due to this fact, the catalytic skill of GC@Cu-Z@HA was additional investigated. The experiment was firstly evaluated by the classical methylene blue (MB) colorimetric technique, by which MB may very well be decoloured by ∙OH, and the manufacturing of ∙OH was judged by whether or not or not there was a lower in absorption. It was noticed that in contrast with the management group, the absorption depth of MB didn’t change considerably whether or not the nanocarrier was incubated with GSH or H2O2 alone, or solely GSH and H2O2 had been combined (Fig. 3E). Comparatively, solely when the nanocarrier was combined with GSH and H2O2 did the absorption of MB lower considerably, indicating that the nanocarrier might catalyze H2O2 to provide ∙OH solely within the presence of GSH. As well as, the manufacturing of ∙OH was additionally exhibited concentration-dependent, that was, the upper focus of nanocarrier, the higher discount of MB absorption (Fig. S9).
Subsequent, to additional confirm the technology of ∙OH, 5,5-dimethyl-1-pyrroline-n-oxide (DMPO) was chosen as particular trapping agent by way of electron spin resonance (ESR) spectroscopy. It was discovered that the attribute 1:2:2:1 sign peak of ∙OH appeared when GC@Cu-Z@HA was combined with GSH and H2O2 (Fig. 3F). In the meantime, terephthalic acid (TA) was used as the precise fluorescent probe to detect the manufacturing of ∙OH. It might react with ∙OH and reworked into 2-hydroxyterephthalic acid, which emitted 435 nm fluorescence underneath 315 nm excitation. In an effort to keep away from the affect of Cur on fluorescence spectrum, the G@Cu-Z@HA with out Cur was used on this experiment. The end result confirmed that there was no fluorescence within the G@Cu-Z@HA and G@Cu-Z@HA + GSH teams (Fig. S10A, B), and when H2O2 was added to the system, solely confirmed very weak fluorescence (Fig. S10C, D), because of the decomposition of H2O2 itself to generate a small quantity of ∙OH. Nonetheless, when GSH and H2O2 had been each added to the G@Cu-Z@HA system, sturdy fluorescence peak appeared (Fig. S10E), and the fluorescence regularly elevated with time. After 10 min, the depth was considerably totally different from these of different experimental teams (Fig. S10F), additional indicating that the Fenton-like response occurred solely when G@Cu-Z@HA was incubated with GSH and H2O2.
Within the proposed design, as NO donor, GSNO might produce NO by the catalysis of copper ion. Due to this fact, Griess reagent was used to detect the discharge kinetic of NO from GC@Cu-Z@HA in weakly acidic atmosphere. The quantity of releasing NO was obtained in response to the usual curve of NO detection equipment (Fig. 3G). The end result revealed that the discharge of NO was positively correlated with the focus of GC@Cu-Z@HA (Fig. 3H), and regularly elevated with the extension of time. As well as, the discharge of chemotherapeutic drug (Cur) in several pH circumstances was additionally explored, and it was discovered that the discharge charge of Cur at pH 6.5 was considerably increased than that of pH 7.4 (Fig. 3I), which additionally implied that the weakly acidic microenvironment in tumor supplied an excellent situation for the degradation of the nanocarrier and the sluggish launch of the drug. All in all, the above end result confirmed that GC@Cu-Z@HA had the property of responsive drug launch in tumor microenvironment, and the launched Cu2+ was diminished to Cu+ by GSH, which subsequently triggered the discharge of NO and the technology of ∙OH. There was little question that the all-in-one multifunctional nanocarrier might possess glorious anti-tumour exercise.
TME-responsive property characterization of GC@Cu-Z@HA nanocarrier. TEM picture of its degradation at totally different circumstances (A); its hydrated particle measurement change at pH 6.5 for 4 h storage (B); its depletion of GSH measured by DTNB at totally different time factors (C) and the corresponding quantitative end result (D); the detection of ∙OH in several therapy circumstances by absorption spectrum (E) and ESR (F); the usual curve of NO detection equipment (G); Griess reagent to detect NO produced by totally different focus of nanocarrier (H); the discharge curve of Cur at totally different pH (I)
In vitro exercise examine of GC@Cu-Z@HA
The blood compatibility of the nanocarrier was an essential index for biosafety analysis. Thus, hemolysis assay was examined by incubating totally different focus of GC@Cu-Z@HA with crimson blood cell for various time. It was discovered that the hemolysis charge was nonetheless decrease than 10% even when the focus of nanocarrier was as excessive as 400 µg/mL after incubation 6 h (Fig. 4A, S11). Because of the distinction of osmotic stress between H2O and PBS, the hemolysis of crimson blood cell within the former was 100%, whereas that within the latter was 0. Smear micrograph of crimson blood cell precipitation confirmed that the morphology of cell handled with nanocarrier was nonetheless common, in step with the end result within the PBS group (Fig. 4B), whereas the cell in ultrapure water was hemolyzed and fragmented, and nearly no cell morphology may very well be noticed. The experiment displayed that the nanocarrier had good blood compatibility and supplied security assurance for subsequent experiment in vivo.
Within the above experiment, it demonstrated that GC@Cu-Z@HA might degrade and launch Cu2+ in mildly acidic resolution, which reacted with GSH to generate Cu+ by way of redox response, after which the NO obtained by ionic catalysis NO donor (GSNO). Moreover, the discharge of NO triggered by copper ion was additionally examined on the mobile stage. Firstly, the cell viability of mouse breast most cancers 4T1 cell at totally different focus of Cu2+, GSNO and Cu2++GSNO was evaluated by MTT assay, and the end result confirmed that the cytotoxicity of the GSNO therapy group was low, and the cell survival charge was nonetheless increased than 80% even when its focus was as excessive as 1 mM (Fig. 4C); for the cell handled with Cu2+ and Cu2++GSNO, it exhibited important focus dependent development, and the cell survival charge was solely 67.96% when the Cu2+ focus reached 1 mM (Fig. 4D), whereas 38.34% in Cu2++GSNO handled group (Fig. 4E), considerably decrease than that of the Cu2+ alone therapy group (p < 0.001). It could be attributable to Cu2+ may very well be diminished by GSH and reworked into Cu+ to provide ∙OH and exerted CDT impact, whereas the cell viability of Cu2++GSNO therapy group additional decreased because of the NO launch by the catalysis of Cu+. Nonetheless, the cell handled with GSNO group couldn’t exert the corresponding therapeutic impact because of the lack of copper ion that inducing the decomposition of GSNO to launch NO.
In the meantime, DAF-FM DA fluorescent probe was additional utilized to detect the manufacturing of NO with totally different therapies. DAF-FM DA might freely cross the cell membrane, and be catalyzed by intracellular lactase to kind DAF-FM, which might react with NO and emit inexperienced fluorescence, whose depth was strengthened with the rise of intracellular NO. As anticipated, apparent NO sign was noticed within the cell incubated with Cu2++GSNO (Fig. S12), resulting from the truth that Cu2+ was diminished to Cu+ by overexpressed GSH in most cancers cell, which triggered the catalytic decomposition of GSNO; whereas nearly no fluorescence sign was detected within the Cu2+ group, and solely few NO was detected within the GSNO group owing to the instability of GSNO, and minor degradation occurred throughout the cell; nevertheless, the fluorescence sign was considerably weaker than that of Cu2++GSNO group because of the lack of NO release-inducing issue. The end result additionally totally demonstrated that the formation of NO relied on the copper ion-catalyzed decomposition response of GSNO.
In an effort to enhance the steadiness and tumor concentrating on of the nanocarrier, HA was modified on the floor of GC@Cu-ZIF-8, which might particularly work together with the overexpressed CD44 receptor on 4T1 cell, and successfully promoted tumor endocytosis of the nanocarrier. Thus, the uptake of nanocarrier by most cancers cell was additional examined. GC@Cu-Z@HA nanocarrier was incubated with 4T1 cell for various time, and the cell nucleus was stained with fluorescent dye DAPI, then the distribution of inexperienced fluorescence of Cur was noticed by confocal fluorescence microscopy. The end result confirmed that weak inexperienced fluorescence appeared within the cytoplasm of 4T1 cell after incubation for 1 h (Fig. 4F), and inexperienced fluorescence additionally appeared within the nucleus after 2 h as a result of Cur was an anti-tumor drug that would act on nucleus [44]; with the prolongation of the incubation time, the inexperienced fluorescence within the cell was additionally enhanced, which implied that increasingly more nanocarrier was endocytosed into the cell. As well as, the quantitative fluorescence depth evaluation of line scan was in step with the results of the confocal fluorescence imaging, and likewise demonstrated the time-dependent increasement. The fluorescence enhanced slowly inside 2 h, considerably rose inside 2 ~ 6 h, and the inexperienced fluorescence of Cur additionally appeared within the nucleus. This was attributed that GC@Cu-Z@HA entered tumor cell by way of receptor-ligand interplay, then degraded in mildly acid atmosphere and launched Cur. Circulation cytometry evaluation was in step with fluorescence imaging end result, which additionally confirmed time-dependent enhancement. The fluorescence elevated slowly inside 2 h, considerably elevated inside 2–6 h (Fig. S13), evaluating with the 0 h, the fluorescence depth modified to 7.35% for 1 h, 28.23% for two h, 63.40% for 4 h, 86.77%, respectively.
Moreover, the depletion of intracellular GSH was additionally evaluated. As a result of GC@Cu-Z@HA nanocarrier would launch Cur with inexperienced fluorescence after being endocytosed into cell, with a view to keep away from its interference on fluorescence imaging, the Cur-free nanocarrier G@Cu-Z@HA was chosen for the related examine. Briefly, ThiolTracker™ Violet was the steady intracellular mercaptan probe, since most free mercaptan in cell represented because the diminished GSH, this probe may very well be utilized to detect intracellular GSH, the stronger inexperienced fluorescence, the upper relative GSH content material. It was noticed that the brilliant inexperienced fluorescence appeared within the untreated cell (Fig. 4G), whereas that within the cell was significantly decreased after incubation with G@Cu-Z@HA, and the depth of inexperienced fluorescence was negatively correlated with the focus of nanocarrier. This was conjectured when G@Cu-Z@HA was endocytosed into tumor cell, the launched Cu2+ might successfully deplete GSH after acid degradation. As well as, DCFH-DA and DAF-FM DA fluorescent probes had been used for evaluating the manufacturing of intracellular ROS and NO. DCFH-DA might freely cross the cell membrane, and it was hydrolyzed into DCFH by lactase after getting into the cell, whereas intracellular ROS might oxidize non-fluorescent DCFH into DCF with inexperienced fluorescence, the rise of intracellular ROS would result in the gradual enhancement of fluorescence. The experimental end result demonstrated that the quantity of intracellular ROS was certainly positively correlated with the focus of G@Cu-Z@HA (Fig. 4H), when the cell was handled with the low focus of G@Cu-Z@HA, weak inexperienced fluorescence was noticed, and the inexperienced fluorescence was getting stronger and stronger with the increasement of focus, nevertheless, the inexperienced fluorescence may very well be hardly noticed within the untreated cell. The stream cytometry evaluation revealed that the technology of ROS was principally in step with fluorescence imaging, the fluorescence of DCF was enhanced with the rise of nanocarrier focus (Fig. S14). This was resulting from the truth that Cu2+ was reworked into Cu+ within the strategy of depleting GSH, which acted because the Fenton-like reagent and catalyzed excessive focus of H2O2 to generate ∙OH in tumor cell, and the improved fluorescence of ROS may very well be noticed. Nonetheless, GSH was an important lowering substance in cell, it might scavenge ROS to guard cell from injury, thus weakening the efficacy of CDT. Within the design, the nanocarrier possessed the power of producing ROS whereas depleting GSH, and the most cancers cell misplaced the safety of GSH, and the redox homeostasis was disrupted, thus exacerbating oxidative stress and inducing the injury of tumor cell. As well as, fluorescence imaging of intracellular NO confirmed the same sample (Fig. 4I). In contrast with the untreated cell, the intracellular inexperienced fluorescence turned stronger and stronger with the rise of the nanocarrier focus after incubation with G@Cu-Z@HA, and the depth of intracellular fluorescence underneath excessive focus of G@Cu-Z@HA was considerably brighter than the opposite teams. Equally, the results of intracellular NO analyzed by stream cytometry additionally elevated with the focus of nanocarrier (Fig. S15). The above end result totally mirrored that nanocarrier might successfully deplete intracellular GSH and exert chemodynamic impact to provide ROS and launch NO.
Biocompatibility analysis of nanocarrier and fluorescence imaging of 4T1 cell incubated with nanocarrier. White gentle imaging and hemolysis charge of crimson blood cell after incubation with totally different focus of GC@Cu-Z@HA (A), and the smear micrograph of crimson blood cell (B); MTT assay of 4T1 cell handled with totally different focus of GSNO (C), Cu2+ (D) and Cu2++GSNO (E); fluorescence imaging and quantitative evaluation of the uptake of GC@Cu-Z@HA by 4T1 cell at totally different time factors (F); fluorescence imaging of intracellular GSH (G), ROS (H) and NO (I) after therapy with totally different focus of G@Cu-Z@HA
Lately, the multicellular tumor sphere mannequin (MCTS), as a classical tumor cell tradition mannequin in vitro, supplied an excellent simulation for exploring drug impact within the tumor microenvironment [45]. For this objective, the three-dimensional (3D) MCTS of 4T1 cell was constructed, then GC@Cu-Z@HA was incubated to analyze the labeling impact of nanocarrier on MCTS. The end result revealed that after incubation for six h, the fluorescence produced by GC@Cu-Z@HA might nonetheless be clearly noticed within the core of MCTS, indicating that the nanocarrier was in a position to label the multicellular tumor sphere effectively (Fig. 5A). The entire and orderly operation of cell cycle was regulated by a wide range of molecular sign. Earlier research confirmed that Cur might induce cycle arrest by selling the expression of cyclin kinase inhibitor [42]. In an effort to discover the impact of nanocarrier on the cell cycle of tumor cell, the distribution of cell cycle was analyzed by stream cytometry after therapy with totally different focus of GC@Cu-Z@HA nanocarrier. The end result confirmed that the untreated tumor cell exhibited the cell cycle distribution of 46.1% within the G0/G1-phase, 43.9% within the S-phase, and eight.01% within the G2/M-phase (Fig. 5B); for low focus nanocarrier, the proportion of G0/G1-phase decreased, S-phase barely elevated, and G2/M-phase had little change; nevertheless, after the tumor cell was handled with the excessive focus of nanocarrier, the chances of G0/G1-phase and G2/M-phase had been considerably decreased, whereas that of S-phase was notably elevated, indicating that GC@Cu-Z@HA nanocarrier might successfully arrest the cell cycle of tumor cell within the S-phase, and the proportion of cell arrested within the S-phase elevated with the growing focus of nanocarrier (Fig. 5C). This discovering confirmed that after the tumor cell was handled with nanocarrier, considerable cell was arrested within the S-phase, indicating that the synthesis of DNA and protein was obstructed, thus the proliferation of tumor cell could be inhibited.
Then, the synergistic therapeutic impact of CDT, NO remedy and chemotherapy on 4T1 cell was additionally explored by MTT assay. The end result confirmed that every one 4 sorts of nanocarriers exhibited apparent cytotoxicity with the growing focus (Fig. 5D), and the cell viability of GC@Cu-Z@HA therapy group was considerably decrease than these of different teams. When the focus of nanocarrier was 50 µg/mL, the cell survival charge of the synergistic therapy group (GC@Cu-Z@HA) was solely 23.06%, decrease than 39.38% within the CDT mixed with chemotherapy therapy group (C@Cu-Z@HA) and 66.91% within the CDT mixed with NO therapy group (G@Cu-Z@HA) (p < 0.01), and considerably decrease than 79.18% within the CDT particular person therapy group (Cu-ZIF-8@HA) (p < 0.001). The end result additionally confirmed that GC@Cu-Z@HA nanocarrier exhibited glorious synergistic therapeutic impact of CDT, NO remedy and chemotherapy, and considerably higher than single or twin mode remedy.
Moreover, the cytotoxicity of GC@Cu-Z@HA nanocarrier on mouse embryonic fibroblast 3T3 cell and human breast most cancers MDA-MB-231 cell was investigated by MTT assay. It was discovered that the survival charge of 3T3 cell was 95.60% even the nanocarrier focus reached 50 µg/mL, implying that the nanocarrier possessed low cytotoxicity and good biocompatibility for regular cell. Whereas the survival charge of MDA-MB-231 cell offered considerably concentration-dependent, and solely 29.53% when the nanocarrier focus was 50 µg/mL (Fig. 5E). This was resulting from the truth that HA particularly interacted with the CD44 receptor overexpressed on MDA-MB-231 tumor cell evaluating with the conventional cell, then extra nanocarrier was endocytosed into the tumor cell. In the meantime, the weakly acidic, and comparatively excessive content material of GSH and H2O2 additionally supplied good situation for killing most cancers cell by built-in multifunctional nanocarrier.
As well as, MDA-MB-231 and 4T1 cells with excessive expression of CD44 receptor had been chosen to additional confirm the synergistic therapeutic impact of GC@Cu-Z@HA nanocarrier, and Calcein-AM and propidium iodide (PI) co-staining was carried out on the cells after totally different therapies. Calcein-AM might emit inexperienced fluorescence after the AM group was eliminated by way of the esterase motion in residing cell; PI exhibited crimson fluorescence in useless cell because of the growing membrane permeability, which might imbed within the DNA double helix after reaching the nucleus. The end result displayed that the untreated 4T1 and MDA-MB-231 cells exhibited sturdy inexperienced fluorescence and nearly no crimson fluorescence (Fig. 5F, S16). Whereas the cell handled with Cu-ZIF-8@HA confirmed inexperienced fluorescence accompanied by a number of crimson fluorescence, which was attributed to the manufacturing of ∙OH by way of Fenton-like response of Cu+ generated by the redox response of Cu2+ and GSH, resulting in a sure diploma of apoptosis resulting from CDT; whereas extra crimson fluorescence appeared in G@Cu-Z@HA-treated cell in comparison with the Cu-ZIF-8@HA group, as a result of Cu+ additionally triggered the discharge of NO from GSNO, ensuing within the superposition of CDT and NO remedy; and the crimson fluorescence of C@Cu-Z@HA-treated cell was additional enhanced, indicating that the impact of chemotherapy was superior to that of NO therapy, which was additionally in step with the earlier results of MTT assay; in distinction, many of the cell handled with GC@Cu-Z@HA confirmed vibrant crimson fluorescence with very weak inexperienced fluorescence, indicating that the overwhelming majority of cell died. These knowledge additional confirmed that the synergistic killing impact of CDT, NO remedy and chemotherapy was the strongest.
Mitochondrial membrane potential was the electrochemical gradient shaped by the uneven distribution of proton on each facet of the membrane, and it was additionally one of many essential indexes to guage mitochondrial operate. Underneath the conventional physiological situation, the JC-1 dye would enter the mitochondria as polymer (JC-1 mixture), exhibiting crimson fluorescence; whereas apoptosis occurred, the mitochondrial membrane potential decreased because of the depolarization, and JC-1 existed within the cytoplasm as monomer (JC-1 monomer), exhibiting inexperienced fluorescence. Herin, the membrane potential of mitochondria was measured after the cell was handled with totally different nanocarrier. It was confirmed that sturdy crimson fluorescence and weak inexperienced fluorescence appeared within the untreated 4T1 cell (Fig. 5G), whereas the crimson fluorescence was weakened and the inexperienced fluorescence was barely enhanced in CDT group (Cu-ZIF-8@HA), as a result of ∙OH was produced after Cu2+ depleting GSH, thus disrupted the redox stability and precipitated change in mitochondrial membrane potential; whereas the crimson fluorescence was sequentially weakened and inexperienced fluorescence was sequentially enhanced within the CDT and NO mixed therapy group (G@Cu-Z@HA), and the CDT and chemotherapy therapy group (C@Cu-Z@HA); the cell within the three-mode synergistic therapy group (GC@Cu-Z@HA) exhibited the weakest crimson fluorescence and the strongest inexperienced fluorescence, suggesting that almost all mitochondria was destroyed. The same phenomenon was noticed for a similar therapy of MDA-MB-231 cell (Fig. S17), which additionally additional verified that the diploma of apoptosis within the synergistic therapy group was a lot increased than these within the different therapy teams. As well as, Hoechst 33,342 was a type of cell membrane permeable agent that stained nuclear DNA and emitted blue fluorescence after binding AT-rich sequence in double-stranded DNA, and its fluorescence depth was brighter in apoptotic cell than in regular cell because of the considerably growing membrane permeability of the previous. Due to this fact, GC@Cu-Z@HA nanocarrier was incubated with 4T1 cell for various time, and stained with Hoechst 33,342, it confirmed that the cell appeared weak blue fluorescence earlier than therapy (Fig. S18), whereas the blue fluorescence was regularly elevated with the prolongation of incubation time, and nearly the entire nuclei confirmed vibrant blue fluorescence after 6 h. This was additionally in step with the earlier results of cell uptake, that GC@Cu-Z@HA might enter cell and play its corresponding function, which additional verified that GC@Cu-Z@HA nanocarrier might successfully induce apoptosis.
The labeling of MCTS and killing impact on cell by nanocarrier. The intense subject and confocal fluorescence picture of MCTS labeled by GC@Cu-Z@HA (A); the cell cycle distribution (B) and proportion (C) of 4T1 cell handled with totally different focus of GC@Cu-Z@HA by stream cytometry; the survival charge of 4T1 cell with totally different therapies by MTT assay (D); the cytotoxicity of 3T3 and MDA-MB-231 cells handled by totally different focus of GC@Cu-Z@HA (E); the fluorescence imaging of Calcein-AM and PI staining MDA-MB-231 and 4T1 cells (F); the fluorescence imaging of mitochondrial membrane potential of 4T1 cell with totally different therapies detecting by JC-1 dye (G); **: p < 0.01, ***: p < 0.001
In vivo biosafety analysis
It was essential for nanocarrier to guage the biosafety on mouse by blood biochemical evaluation. Wholesome 6-week-old feminine Balb/C mice had been randomly divided into three teams (n = 5), two teams had been used to guage the short-term toxicity by single injection and cumulative toxicity by a number of injections of GC@Cu-Z@HA nanocarrier (each two days for a complete of three injections) respectively, the third group was injected with the equal quantity of PBS as management. On the finish of remark interval, the blood of the mice was collected for blood cell evaluation. The end result confirmed that in contrast with the management group, there was no important distinction in white blood cell (WBC), crimson blood cell (RBC), hemoglobin (HGB) and platelet (PLT) in wholesome mice after injection with GC@Cu-Z@HA nanocarrier inside 28 days (Fig. S19A–D), which demonstrated that the nanocarrier had good blood compatibility. Then, the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) had been detected for liver operate index, blood urea nitrogen (BUN) and creatinine (CR) for kidney operate index. It confirmed ALT and AST exhibited no apparent change between all teams (Fig. S19E, F). However BUN and CR decreased barely with growing variety of injections (Fig. S19G, H), which could be the conventional response of the physique to overseas substance, however these indexes had been nonetheless throughout the regular reference vary (BUN: 9.75–22.71 mg/dL, CR: 10.90-118.07 µM/L). The change of organ index and H&E part of mice in every group had been additionally used to find out whether or not GC@Cu-Z@HA nanocarrier might trigger organ lesion. In the meantime, the organ index was an essential indicator to guage the well being of organ, which was the ratio of organ weight to physique weight in mouse, and the ratio was comparatively fixed in regular mouse, as soon as the organ was broken, the organ index would additionally change. The end result demonstrated that in contrast with the management group, there was no important distinction within the coronary heart, liver, spleen and kidney indexes with single injection and a number of injections (Fig. S19I-L), they usually had been additionally throughout the regular vary. What’s extra, the results of H&E part displayed that the tissue construction of mice injected with GC@Cu-Z@HA nanocarrier single or a number of occasions had been intact with out apparent damage or pathological change (Fig. S19M). In abstract, GC@Cu-Z@HA nanocarrier had good low toxicity and glorious biosafety, which was conducive to subsequent utility in vivo.
In vivo imaging of GC@Cu-Z@HA
Based mostly on the superb security in vivo, the continual fluorescence imaging monitoring of tumor-bearing mice injected with nanocarrier was carried out. The 4T1 cell was chosen because the mannequin cell and inoculated on the primary pair of breast pad on the left facet of feminine Balb/C mice to assemble orthotopic breast most cancers mannequin. The end result confirmed that no fluorescent sign was detected on the tumor website earlier than injection (Fig. S20). Whereas the fluorescence sign of Cur appeared clearly on the tumor after intratumoral injection of GC@Cu-Z@HA, and the fluorescence regularly weakened till disappeared with the prolongation of time, as a result of the Cur loaded within the nanocarrier entered the tumor website with the injection, after which regularly degraded within the tumor microenvironment, thus the Cur additionally unfold across the tumor.
As well as, Mitochondrial metabolism is essential for tumor proliferation, tumor survival, and metastasis. Most research confirmed that organismal metabolic course of strongly relied on the involvement of NAD(P)H and flavin adenine dinucleotide (FAD), and their content material and relative change mirrored the metabolic stage of tissue [46]. NAD(P)H and FAD are two autofluorescent coenzymes within the mitochondrial respiratory chain that can be utilized as endogenous fluorescent metabolic indicators to characterise the state of mitochondrial power metabolism. In our design, Cu2+ might deplete GSH and alter the relative content material of NADPH on the tumor website, and disrupted GSH/GSSG and NADPH/NADP+ redox stability, which in flip affected the NADPH/FAD power metabolism and redox state throughout the tumour. That’s to say, the depletion of GSH additionally causes the lower of NADPH content material, which implies that the recycle of NADPH/FAD is disrupted, resulting in a collapse in ATP manufacturing. This collapse in flip disrupts the power provide and successfully inhibits mobile respiration, thus disrupts redox and metabolic homeostasis within the tumor cell. Due to this fact, underneath deep hypothermia induced by liquid nitrogen, the improved autofluorescence depth of NADH/FAD in tumor was thought to be an analysis index to observe the change of autofluorescence depth of NADPH/FAD and its redox ratio NADPH/(NADPH + FAD), which comprehensively evaluated the standing of intratumoural redox and power metabolism. Since NADPH and FAD each had autofluorescence, with a view to keep away from the affect of Cur on fluorescence sign, the nanocarrier with out Cur, G@Cu-Z@HA, was chosen for this experiment. The 2D/3D outcomes confirmed that earlier than injection of G@Cu-Z@HA, a considerable amount of NADPH within the tumor tissue (Fig. 6A, S21), and its fluorescence depth remained the identical. After injection with nanocarrier for 0.1 h, the fluorescence depth of NADPH regularly weakened with the extension of time, whereas the distribution of FAD was simply the other development, and the distribution of the redox ratio additionally coincided with the quantity of NADPH. At 12 h, the fluorescence sign of NADPH was the weakest, whereas that of FAD was the strongest, and the NADPH/(NADPH + FAD) worth was the bottom, indicating that the tumor tissue was in sturdy oxidation state. In the meantime, the regional depth evaluation of NADPH redox additional verified the change of NADPH on the tumor website over time (Fig. 6B). It is also seen from the histogram evaluation that the NADPH/(NADPH + FAD) worth regularly weakened from 0.1 to 12 h, and the redox ratio was the smallest at 12 h. This was resulting from the truth that the launched of Cu2+ by the degradation of the nanocarrier G@Cu-Z@HA within the tumor regularly depleted GSH, and likewise precipitated the lower of NADPH, then in flip affected the redox stability of the tumor, indicating that the nanocarrier might certainly successfully regulate endogenous power metabolism of the tumor.
In vivo synergistic anti-tumor examine of GC@Cu-Z@HA
Then, with a view to consider the synergistic anti-tumor impact of GC@Cu-Z@HA in vivo, the therapy rule for orthotopic breast most cancers was designed (Fig. 7A). For the constructed orthotopic breast most cancers mannequin mice, when the tumor quantity reached about 100 mm3, the tumor-bearing mice had been randomly divided into 5 teams: (I) PBS, (II) Cu-ZIF-8@HA, (III) G@Cu-Z@HA, (IV) C@Cu-Z@HA, (V) GC@Cu-Z@HA. On day 0, 3 and 6, totally different nanocarrier was injected into mice by intratumoral injection. In the course of the experiment, the change of physique weight and tumor quantity of mice in every group had been measured each different day, in order to comprehensively consider the tumor therapy impact. It was discovered that the common physique weight of mice in experiment group exhibited no obvious change in contrast with the PBS group inside 14 days (Fig. 7B); and the H&E staining results of coronary heart, liver, spleen, lung, kidney, and gut of the mice in every therapy group additionally confirmed that the organ construction remained intact with out apparent injury or pathological change on the finish of remark interval (Fig. S22), indicating that the nanocarrier didn’t trigger evident injury to the mice. And the tumor quantity of mice in PBS group grew quickly with time (Fig. 7C); whereas the expansion charge of tumor quantity in Cu-ZIF-8@HA group was considerably inhibited, which was attributable to the CDT impact and the depleting of excessive focus of GSH by Cu-ZIF-8@HA on the tumor website, which additional delayed the speedy development of tumor; the tumor quantity of tumor-bearing mice in G@Cu-Z@HA and C@Cu-Z@HA teams was additional inhibited, resulting from CDT mixed with NO remedy and CDT mixed with chemotherapy respectively, indicating that dual-mode synergistic remedy had stronger tumor killing impact than single remedy. The GC@Cu-Z@HA group confirmed one of the best inhibitory impact, which was higher than the 2 dual-mode mixed therapy group (p < 0.05), and considerably higher than the only therapy group (p < 0.001).
Lastly, on the finish of therapy, the mice of every group had been euthanized, the tumors had been stripped and weighed for high quality evaluation, it was discovered the development was in step with the results of tumor quantity (Fig. 7D), indicating that the GC@Cu-Z@HA synergistic therapy group exhibited probably the most glorious efficacy and the very best tumor inhibition charge of 56.38% (Fig. S23), which was extra higher than that of Cu-ZIF-8@HA group (p < 0.01) and G@Cu-Z@HA group (p < 0.05). Evaluating totally different experimental teams of mice earlier than and after therapy, it is also seen that GC@Cu-Z@HA confirmed one of the best therapeutic impact (Fig. 7E). This was resulting from the truth that the multifunctional GC@Cu-Z@HA may very well be delivered to tumor cell by way of HA-mediated focused supply, then degraded in response to the tumor microenvironment, and the launched Cu2+ depleted intratumoural overexpressed GSH and likewise generated extremely poisonous ∙OH by way of Fenton-like response, thus disturbing the redox stability of most cancers cell and enhancing the efficacy of CDT. In the meantime, it might additionally set off the discharge of NO in situ to dilate blood vessel, improve drug penetration on the tumor website and induce cell cycle arrest, ensuing within the apparent tumor cell killing impact.
In the meantime, the tumor tissue of mice in several therapy teams was obtained for H&E, TUNEL and Ki67 staining with a view to totally consider the tumor therapy impact. The results of H&E confirmed that there was nearly no apoptosis within the management group because of the quick cell proliferation and dense construction of tumor cell (Fig. 7F), whereas a sure diploma of cell shrinkage appeared within the CDT therapy group (Cu-ZIF-8@HA), and the cell apoptosis was additional elevated after therapy of CDT combing with NO remedy (G@Cu-Z@HA) or chemotherapy (C@Cu-Z@HA), whereas important wrinkling of tumor nucleus and extreme destruction of cell construction had been noticed within the GC@Cu-Z@HA group, implying the presence of numerous apoptotic cell. As well as, TNUEL immunofluorescence staining was additionally carried out, by which the inexperienced fluorescence sign represented the diploma of DNA injury. It was discovered that the brightest inexperienced fluorescence appeared within the GC@Cu-Z@HA group (Fig. 7G), indicating that the DNA injury was extraordinarily critical after synergistic therapy. The crimson fluorescence of Ki67 immunofluorescence staining represented the cell proliferation charge, and the end result demonstrated that the strongest fluorescence sign appeared within the PBS group (Fig. 7H, S24), whereas the weakest fluorescence sign was noticed within the GC@Cu-Z@HA therapy group, additional indicating that the built-in multifunctional nanocarrier might successfully enhance the therapeutic impact of tumor.
Moreover, the operate of NO on tumor vasculature was to dilate vascular clean muscle and improve vascular permeability on the tumor website. In the course of the synergistic therapy of tumor with the nanocarrier, whether or not the therapy with the NO-generated nanocarrier might result in vasodilation of tumor vasculature was additionally exploited, utilizing the dye-labeled CD31 antibody to find blood vessel within the tumor, and immunofluorescence staining was carried out on totally different layers of tumor tissue within the PBS, Cu-ZIF-8@HA, G@Cu-Z@HA therapy teams. Because the tumor tissue comprise blood vessel, the crimson fluorescence sign was additionally noticed within the center tumor tissue of the PBS and Cu-ZIF-8@HA teams (Fig. 7I), whereas the G@Cu-Z@HA-treated group confirmed stronger fluorescent sign, and the variety of vasodilated blood vessel additionally elevated considerably (the white arrow represented the dilated tumor blood vessel), and the vascular endothelial tissue was extra intact. The same end result was noticed within the staining of blood vessel within the outer and inside layer of the tumor tissue (Fig. S25). This additionally indicated that NO generated by the copper ion-catalyzed decomposition response of GSNO might successfully promote vasodilation, thereby inducing vascular density to extend in tumor tissue, ensuing within the elevated blood stream and drug supply within the blood vessel, thus facilitated the penetration of the drug within the tumor website, which additionally additional confirmed that NO performed an essential function in vasodilation. In the meantime, the impact of NO on the deep penetration of Cur into the tumor website was additionally explored by performing frozen part of tumor tissue with totally different therapies after peritumoral injection. Confocal fluorescence microscopy was used to look at the intra-tumoral distribution of inexperienced fluorescence of Cur contained within the nanocarrier in tumor, and cell nuclei was stained with fluorescent dye DAPI. The end result confirmed that within the mice handled with the free Cur, solely a really small quantity of fluorescence was detected all through the tumor and primarily distributed across the tumor (Fig. 7J). Against this, C@Cu-Z@HA handled tumor exhibited stronger fluorescence sign all through the tumor tissue, however the fluorescence was primarily distributed across the tumor tissue than that handled with free Cur. Whereas GC@Cu-Z@HA handled tumor offered the strongest fluorescence, and vibrant fluorescence may very well be noticed in the entire tumor tissue, and the fluorescence was extra broadly distributed within the tumor than that different therapy teams, indicating that the drug penetration impact was stronger, which additional highlighted the extraordinarily essential function of NO within the deep penetration of drug into the tumour tissue. This was resulting from the truth that free drug Cur was small molecule that would freely penetrate tumor tissue and thus been quickly metabolized and excreted from the physique. As nanocarrier, C@Cu-Z@HA and GC@Cu-Z@HA would accumulate extra in tumor website by way of EPR impact, whereas the manufacturing of NO in situ by GC@Cu-Z@HA successfully promoted the deep penetration of the drug within the tumour tissue.
In abstract, GC@Cu-Z@HA nanocarrier offered the power of TME-response and performed the therapeutic impact of CDT together with NO remedy and chemotherapy. The synergistic anti-tumor efficacy was considerably higher than these of single and two-modality mixed therapies, and attained glorious therapeutic efficacy with out important toxicity in vivo, which supplied a brand new concept for designing environment friendly built-in tumor therapy technique.
In situ breast most cancers therapy of nanocarrier. Schematic illustration of therapy rule for tumor (A); the physique weight (B), tumor quantity (C) and tumor weight evaluation (D) of 4T1 orthotopic breast most cancers with totally different therapies; {photograph} of mice earlier than and after with totally different therapies (E); H&E staining and enlarged picture (F), TUNEL (G), Ki67 (H) and CD31 (I) immunofluorescence staining of tumor after totally different therapies; confocal fluorescence picture of tumor tissue part after peritumoral injection (J); n = 5, *: p < 0.05, **: p < 0.01, ***: p < 0.001