Brokers for tumor imaging and remedy have been supposed to own totally different intratumoral behaviors. LABO demonstrating viscosity and concentration-dependant intratumoral in-situ self-assembly would possibly meet the necessities.
Synthesis and characterizations
Synthesis of LABO and 18F-LABO
The synthesis of LABO was proven within the scheme 1. The BODIPY-3 was three-step synthesized from 2,4-dimethyl-pyrrole and 4-(hydroxymethyl)benzaldehyde. After condensation with 4-(bis(4-methoxyphenyl)amino)benzaldehyde, and glycosylation with LA, the aimed product LABO was obtained. The entire merchandise have been characterised by mass spectrum and nuclear magnetic resonance, as proven in Fig S1–S9. 18F-LABO was then synthesized by 18–19 F trade beneath the catalysis of SnCl4 because the protocols reported earlier than [23, 24]. As a isotope labeled compounds of LABO, 18F-LABO possessed comparable chemical properties (hydrophilicity and polarity e.g.) and chemical exercise to LABO, which may very well be used to analyze the in vivo behaviors of LABO, however may also show just a little variations [25, 26]. For instance, the chemical response price is likely to be influenced because of the molecular weight change, which was often known as kinetic isotope impact (KIE). In the meantime, some bodily properties may also change corresponding to vapor density, diffusion velocity and magnetic resonance conduct (18F confirmed no magnetic resonance sign).
Characterizations
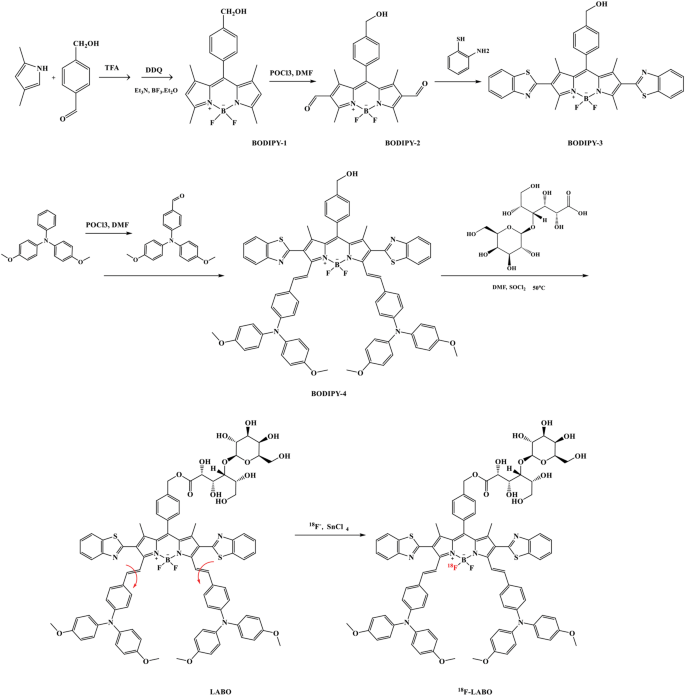
The optical behaviors of LABO in water/methanol blended resolution was firstly investigated, as proven in Fig. 2a. When dissolved in pure methanol, LABO confirmed absorption peak at 570 nm, and its absorption at 650 nm was inhibited. Nevertheless, with the elevated water fraction within the blended resolution, the absorption at 650 nm was elevated, and reached peak when water fraction reached 70%, which could resulted from the free rotation restriction of the double bonds of LABO. Identical state of affairs was additionally absorbed when UV absorption spectrum was measured in glycerinum/methanol blended system, as proven in Fig. 2b. With the elevated fraction of glycerinum, the system viscosity was enhanced and the absorption at 650 nm was additionally elevated, which arrived at peak when glycerinum fraction reached 50%. For fluorescence emitting spectrum, as confirmed in Fig. 2c, LABO was non-fluorescent at 720 nm when dissolved in methanol. Nevertheless, with the improved system viscosity by rising glycerinum fraction, the fluorescence at 720 was additionally enhanced. The particular spectrum behaviors of LABO proved our assumptions that the in-situ aggregation of LABO was a form of J-aggregation that may occur between conjugated molecules with poor coplanarity [10]. On the one hand, LABO possessed free rotation double bonds, which not solely diminished its conjugated system and make the absorption band blue shifted, but additionally allowed photon-excited LABO to launch the power in a non-radiative pathway and led to the fluorescence inhibition. When viscosity enhanced, the free rotation of double bond was blocked, and the molecular rigidity was enhanced. The enlarged and stuck conjugated programs of LABO made the UV absorption band pink shifted, and allowed excited electrons to return to the bottom state in a radioactive pathway, thus resulting in the fluorescence restoration [27]. However, the LA tail of LABO generated intermolecular steric hindrance and diminished the intermolecular coplanarity. Because of this, the structure-fixed LABO was unable to type π-π stacking in a head-to-tail mannequin (full stacking), specifically H-aggregation, by which the molecules have been intently organized, and resulted to the absorption band blue shift and fluorescence quenching. LABO tended to type π-π stacking in a head-to-head or tail-to-tail mannequin (partial stacking), specifically J-aggregation [28]. J-aggregation with robust electron coupling may trigger the absorption band pink shift and fluorescence enhancement, which have additionally been noticed in our optical spectrum investigation [29].
To instantly observe the LABO NPs, TEM imaging was carried out, and LABO NPs have been offered because the nanoparticles with 100–200 nm diameters, as confirmed in Fig. 2d.
As a BODIPY by-product, LABO NPs was thought-about to be phototoxic and confirmed potential as a photodynamic remedy agent [10, 30, 31]. Therefore, the irradiation-induced reactive oxygen species (ROS) era was monitored by ROS probe (DCFH), as proven in Fig. 2e. LABO NPs demonstrated little ROS era when incubated with DCFH in the dead of night. Nevertheless, When irradiation was administrated for 60 s, evident ROS era was noticed, as indicated by the improved fluorescence of DCF, the ROS oxidative product of DCFH. In the meantime, the ROS outbreak was noticed the the irradiation time was extended to fifteen min, and the fluorescent depth was enhanced by almost 50 occasions, indicating the robust phototoxicity of LABO NPs.
18F labeled LABO (18F-LABO) was synthesized through 18–19 F trade of LABO. The 18F labeling price was 83%, as proven in Fig. 2f, on which the free 18F− and 18F-LABO appeared at about 2.6 min and 5.7 on excessive efficiency liquid chromatography that outfitted with radioactivity detector (radio-HPLC). LABO and 18F-LABO have been co-injected into radio-HPLC to confirmed the id of 18F-LABO, Fig. 2g. The UV peak of LABO was just a little forward of the radioactive peak of 18F-LABO (15–20 s) because of the sequence connection of the UV and radioactivity detector. After formulation, 18F-LABO was injected in radio-HPLC, and no chemical or radiochemical impurities was discovered within the radio-HPLC, Fig. 2h. Solely just a little unlabeled LABO appeared in radio-HPLC, and the specificity exercise was 52.46 Ci/mmol. The in vitro stability of 18F-LABO was evaluated in mouse serum and PBS (pH = 7.4).18F-LABO remained greater than 90% intact after 60 min incubation. After 240 min incubation (greater than 2 half-life), greater than 80% 18F-LABO stayed nonetheless, as proven in Fig. 2f.
Synthesis and characterization of LABO, LABO NPs and 18F-LABO (a: UV spectrum modifications of LABO in water/methanol resolution, in every titration water fraction was enhanced ~ 10%; b:UV spectrum modifications of LABO in glycerinum/methanol resolution, in every titration glycerinum fraction was enhanced ~ 12%; c: fluorescent spectrum modifications of LABO in glycerinum/methanol resolution, in every titration glycerinum fraction was enhanced ~ 7%; d: TEM imaging of LABO NPs; e: ROS induced fluorescence of DCFH-DA; f: labeling price of 18F-LABO, g: co-injection of LABO and 18F-LABO; h: radio-HPLC evaluation of 18F-LABO after formulation, i: stability of 18F-LABO in PBS and mouse serum)
Cell assays
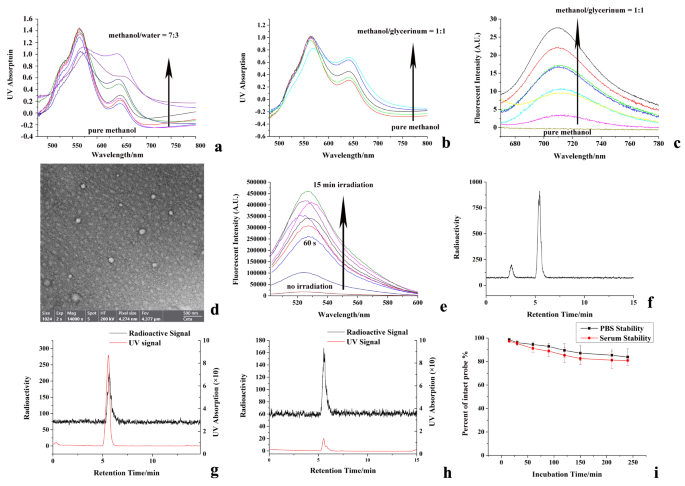
To visualise the ASGPR specificity and intracellular ROS era, the intracellular fluorescence colocalization and DCF fluorescent imaging have been carried out. 2’,7’-Dichlorodihydrofluorescein diacetate (DCFH-DA) have been used because the ROS probe within the cell as a substitute of DCFH. HepG2 cell was incubated with LABO, fluorescence-labeled ASGPR-antibody and DCFH-DA for 15 min, throughout which cell was additionally irradiated. As confirmed in Fig. 3a1−3a4, although cell was dyed by the internalized LABO and confirmed unhappy standing within the vivid subject imaging because of the phototoxicity, shade and tinting power of BODIPY-based LABO, vivid intracellular NIR fluorescence of LABO NPs (3a2) with robust colocalization to fluorescent ASGPR-antibody (3a3) and DCF (3a4) was noticed, not solely manifested the ASGPR specificity of LABO, but additionally show the intracellular ROS era. Nevertheless, when cell was pretreated with LA to dam the ASGPR, the intracellular fluorescence of LABO and fluorescent antibody have been considerably inhibited, resulted to the much less era of ROS and decreased DCF fluorescence, as present in Fig. 3a5−3a8. The cell uptake dynamic was additionally analyzed, and the intracellular fluorescent depth was quantified by imageJ, as proven in Fig. 3b and c. The intracellular fluorescence of LABO NPs was enhanced (from 34.13 ± 3.65 to 58.91 ± 4.12 and 88.01 ± 9.19 as quantified by imageJ) with the extended incubation time from 5 min to 60 min and 120 min, indicating the continual cell uptake and intracellular aggregation of LABO apart from cell efflux like different small molecular drug. The Pearson Colocalization Coefficient was 0.91, 0.87, 0.87, 0.97 and 0.92 at 30 min, 45 min, 60 min, 90 min and 120 min after incubation, respectively. ROS era was additionally improved over incubation time, the intracellular fluorescent depth of DCF was enhanced from 26.11 ± 5.03 to 33.98 ± 5.77 and 49.61 ± 7.12 after 60 and 120 min of incubation, as additionally demonstrated in Fig. 3c.
The phototoxicity of LABO was additionally investigated, as proven in Fig. 3d. LABO confirmed low cytotoxicity in the dead of night. Cell viability remained 89.6 ± 5.9%, 83.12 ± 7.16%, 80.77 ± 6.68% after 24, 48 and 96 h incubation with LBAO (10 µM). Nevertheless, when cell was incubated with LABO beneath irradiation, the cytotoxicity was considerably promoted. Cell viability decreased to 23.8 ± 4.4%, 17.33 ± 6.34% and 11.22 ± 4.83% after 24, 48 and 96 h incubation with LBAO (10 µM). The IC50 was 2.12, 1.44 and 0.95 µM for twenty-four, 48 and 96 h incubation, as calculated by GraphPad Prism. Detailed cell viability information was additionally offered in Desk S1.
Cell recognition and specificity of LABO to ASGPR have been then monitored by coincubation 18F-LABO with ASGPR-positive HepG2 cells. Because the leads to Fig. 3e demonstrated, 18F-LABO confirmed speedy binding to HepG2 cells inside 30 min, and 5.72 ± 0.77% radioactivity was noticed within the cell. Cell radioactivity accumulation elevated with the extended incubation time, and reached the height of 8.05 ± 1.33% at 90 min after incubation. Extending incubation time to 120 min confirmed just a little assist to the radioactivity accumulation (8.32 ± 1.52%). Nevertheless, when cell was pretreated with LA to dam the ASGPR, 18F-LABO binding was considerably inhibited (1.46 ± 0.23% at 30 min and three.03 ± 0.46% at 90 min), indicating the ASGPR specificity of 18F-LABO.
To attain intratumoral in-situ self-assembly, ample cell internalization of LABO after particular recognition and binding to ASGPR was vital. As a outcomes, the cell internalization and efflux of LABO after binding was evaluated by contentious incubation of 18F-LABO-bond HepG2 cell within the recent medium. The efflux ratio was outlined because the radioactivity within the supernate that deriving from the cell efflux to the radioactivity that remained within the cell. The leads to Fig. 3e indicated, as a substitute of overlong binding to the cell surfaces and being quickly washed out, 18F-LABO was capable of penetrate into cells after particular binding to ASGPR. Solely 7.18 ± 3.83% and 19.83 ± 5.68% radioactivity flowed out after one other 60 and 120 min incubation after binding to cell, which mirrored the happy cell internalization and retention of LABO. We thought-about that the acceptable lipophilicity of BODIPY by-product would possibly play important roles within the cell membrane penetration and internalization of 18F-LABO. Robust cell penetration capability and excessive intracellular retention not solely offered the focus situation for the in-situ meeting, but additionally assured the ample irradiation damages from photodynamic remedy.
Cell assays of LABO: a: fluorescent co-localization of LABO, ASGPR receptor and intracellular ROS in free state (a1-a4, vivid filed imaging and fluorescent imaging of LABO, ASGPR antibody and DCF in experimental group cell) or ASGPR blocked state (a5-a8, vivid filed imaging and fluorescent imaging of LABO, ASGPR antibody and DCF in preblocked cell), b/c: dynamic fluorescence change of LABO and DCF within the cell, d: phototoxicity of LABO, e: cell uptake and efflux ration of 18F-LABO
In vivo analysis
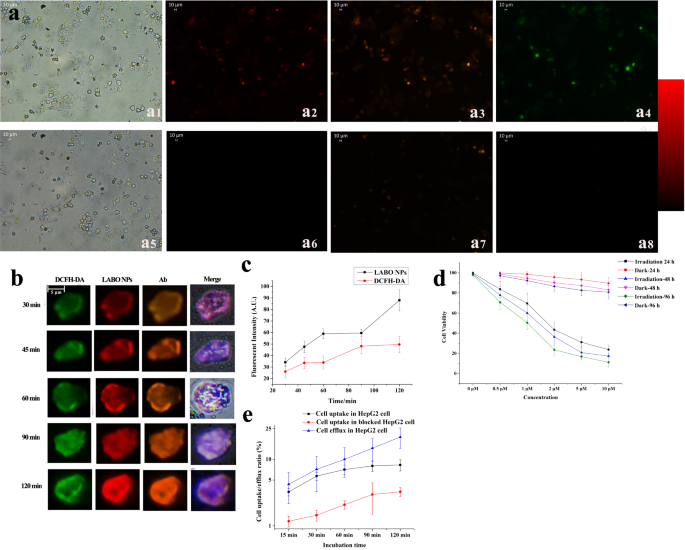
The in vivo analysis of 18F-LABO together with peripheral blood clearance and biodistribution was carried out. As proven in Fig. 4A, the radioactive sign within the blood decreased dramatically from 30.77 ± 6.39percentID/g (15 s) to five.89 ± 0.98percentID/g after 5 min post-injection (p.i). After that, the radioactivity within the blood repeatedly diminished over time, and may very well be nearly cleared from blood 1 h p.i. (0.063 ± 0.012percentID/g).
In the meantime, the biodistribution research in Fig. 4B revealed particular radioactivity within the tumor, which elevated from 0.78 ± 0.23percentID/g 15 min p.i. to 2.32 ± 0.67percentID/g 90 min p.i., and confirmed just a little lower 120 min p.i.(1.87 ± 0.46percentID/g). Therefore, the imaging time level was decided at 90 min p.i. In the meantime, no particular uptake within the muscle (0.41 ± 0.087percentID/g 15 min p.i. and 0.11 ± 0.035percentID/g 90 min p.i.) was noticed. Coronary heart confirmed comparatively excessive radioactivity accumulation at starting (4.76 ± 1.33percentID/g 15 min p.i.) because of the blood pool and myocardium uptake, which diminished over time (0.89 ± 0.32percentID/g 90 min p.i. and 0.42 ± 0.14percentID/g 120 min p.i.). Enhanced radioactive sign was noticed within the liver and kidney (6.74 ± 1.67/4.86 ± 1.54percentID/g for liver/kidney 90 min p.i.), which could resulted from the in vivo defluorination and the metabolic pathway of 18F. Sign in liver and kidney additionally tended to lower (5.89 ± 1.24percentID/g and three.96 ± 0.94percentID/g) 120 min p.i. Overlong retention within the tumor and non-target organs was pointless for PET imaging. 18F-LABO confirmed lack of ability to self-assembly after administrated, and may very well be quickly cleared. The biodistribution information was additionally offered in Desk S2.
To substantiate the totally different intratumoral retention of LABO throughout photodynamic remedy, NIR fluorescent imaging was used to watch the tumor retention of LABO. As confirmed in Fig. 4c, solely background fluorescence was noticed within the tumor 2 h p.i. because of the inadequate LABO NPs era within the tumor. 4 h after injection, weak fluorescence was generated within the tumor, which get brighter over time with the buildup of LABO NPs, and reached peak 12 h p.i., making tumor clearly delineated and distinguished within the photographs. The radiant efficiencies have been 1.18 × 107, 2.43 × 107, 3.66 × 107, 4.23 × 107 and 4.87 × 107 [p/s/cm2/sr]/[µW/cm2] 2, 4, 6, 8 and 12 h p.i. respectively. The fluorescent imaging demonstrated visualized outcomes that the era of LABO NPs considerably enhanced the intratumoral retention of LABO in comparison with 18F-LABO. In the meantime, LABO was non-fluorescent within the non-target (coronary heart, spleen, lung, abdomen and gut), making a low-signal background for fluorescent imaging. Excessive tumor-to-non goal ratio (T/NT) assured ample tumor irradiation damages and minimal undesirable non-target damages throughout photodynamic remedy.
To additional affirm the era and retention of LABO NPs within the tumors at totally different time home windows, TEM imaging was carried out utilizing tumor tissues from mice that handled with LABO (12 h and 18 h after remedy). Regular liver tissue was additionally obtained and imaged 18 h publish administrated. Because the leads to Fig. 4d and Fig S10a-10c indicated, intratumoral in-situ self-assembled nanoparticles have been noticed as black spheres within the tumors at each 12 and 18 h after injection, whereas barely sphere nanoparticles have been noticed in regular liver, as proven in Fig S10d. These outcomes not solely mirrored the lengthy intratumoral retention of LABO NPs, however gave probably the most intuitive evident that LABO may efficiently self-assembled into nanoparticle when subjected to the excessive viscosity environments corresponding to tumor microenvironment and tumor membrane. Nevertheless, its aggregation in regular liver was tougher. We thought-about that solely in some particular circumstances (e.g. mass plasma albumin absorption, irregular accumulation in liver/kidney on account of over excessive focus) would possibly induce the LBAO NPs era. Therefore, the optimum administrated dosage of LABO wanted to be additional explored to boost the therapeutic impact and scale back the liver damages of LABO. Nevertheless, the first experimental evidences that clearly LABO NPs abundance distinction was noticed between tumors and liver have mirrored that the viscosity induced in-situ self-assembly of LABO may very well be a possible instrument to ensured the ample tumor damages whereas assured non-target security throughout photodynamic remedy.
In vivo analysis of LABO and 18F-LABO (A: plasma focus of 18F-LABO over time; B: biodistribution of 18F-LABO, C: fluorescent imaging of HepG2 tumor bearing mice, a-e: 2, 4, 6, 8, 12 h after injection of LABO, D: TEM imaging of intratumoral in-situ self-assembled nanoparticles 12 h publish administration of LABO, which have been pointed by the black arrows)
In vivo PET imaging
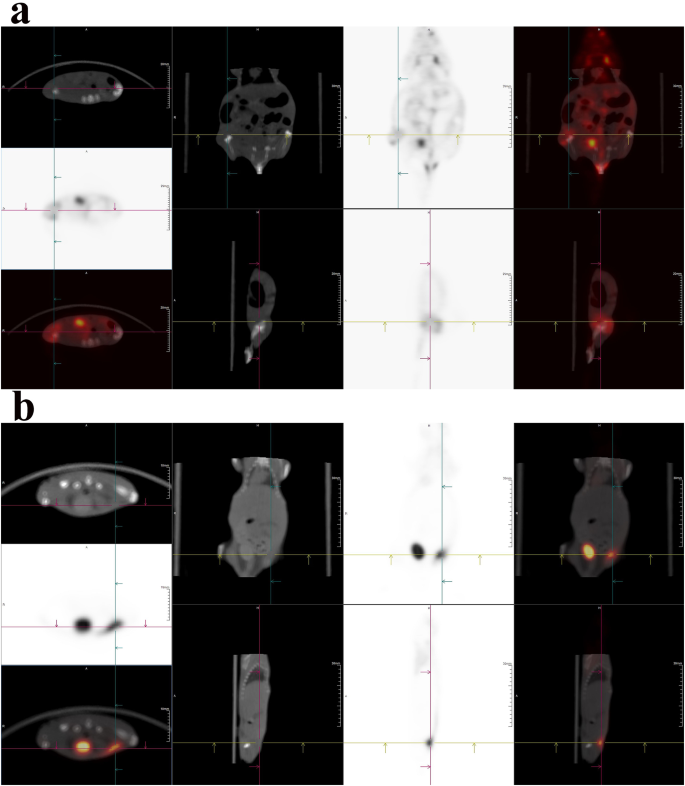
Fluorescent imaging was quick and handy however was restricted by the scattering and tissue penetration depth [32, 33], PET imaging was then carried out primarily based on 18F-LABO because the complement, as proven in Fig. 5a (45 min p.i.) and 5b (90 min p.i). When imaged at 45 min p.i., 18F-LABO demonstrated particular uptake within the tumor, as indicated by the crosshair in Fig. 5a. Tumor may very well be clearly indicated and delineated by the excessive radioactivity accumulation, and the SUVmean was 2.67. In the meantime, non-target organs corresponding to coronary heart, muscle and gut additionally demonstrated 18F-LABO uptake, which diminished the tumor distinction to some extent, but not yield important affect on tumor remark. When imaged as 90 min p.i., the leads to Fig. 5 demonstrated that 18F-LABO may particularly accumulate within the HepG2 tumor because of the ASGPR recognition, and the SUVmean was 4.47. Tumor may very well be clearly distinguished within the photographs, whereas non-target organs together with spleen, muscle, abdomen and gut exhibited low 18F-LABO. Comparatively excessive radioactivity within the coronary heart (SUVmean = 2.68) was noticed because of the blood pool. Excessive radioactive sign within the bladder was noticed, which could consequence from the metabolic pathway or in vivo defluorination. Since PET imaging primarily based on 18F-FDG confirmed limitations for HCC because of the low uptake within the tumor, we thought-about that 18F-LABO demonstrated excessive ASGPR specificity and wonderful HCC localization confirmed nice potentials as a imaging instrument for HCC screening and staging.
Nevertheless, since PET imaging was carried out primarily based on the positron from 18F, whereas fluorescent imaging was carried out primarily based on the fluorescence of LABO NPs that was inhibited on the free stage, it was vital to say that PET imaging and fluorescent imaging would possibly give totally different in vivo data. When administrated, each 18F-LABO and LABO existed because the type of free small molecule, and will topic to sequence of metabolic pathways corresponding to circulation clearance, kidney retention and phagocytosis of kupffer cell in liver. In these conditions, PET imaging may replicate the metabolic pathways of 18F-LABO, whereas fluorescent imaging would possibly present limitations because of the fluorescence inhibition aside from some particular circumstances as we talked about earlier than (mass plasma albumin absorption, irregular accumulation in liver/kidney on account of over excessive focus), which could additionally result in the partial fluorescence restoration and trigger the totally different metabolic conduct between LABO and 18F-LABO. After that, 18F-LABO and LABO may very well be taken in by no-specific organs corresponding to lung, muscle and abdomen, the place PET imaging may additionally offered the in vivo data, however fluorescence of LABO was nonetheless blocked since there was no required situations for self-assembly. As a outcomes, PET imaging outcomes additionally mirrored the uptake and metabolism in non-target organs. Final however not least, when arriving at peritumor/tumor microenvironment (TME) or particularly binding to tumor surfaces, LABO was self-assembled into LABO NPs because of the excessive viscosity of TME and tumor. The nanoscale aggregation of LABO NPs considerably decreased tumor clearance and enhanced intratumoral retention, resulting in the very long time NIR fluorescence remark window and ample phototoxicity. For 18F-LABO, it was unable to self-assembled into 18F-LABO NPs within the TME or tumors on account of its extraordinarily low chemical equal. Because of this, it’s tumor retention time was a lot shorter than than of LABO NPs, and was cleared quickly from tumor to keep away from pointless radiation damages. In abstract, we thought-about that fluorescent and PET imaging may play totally different roles and provides complementary in vivo data when investigating retention and metabolism in numerous organs.
Antitumor results analysis
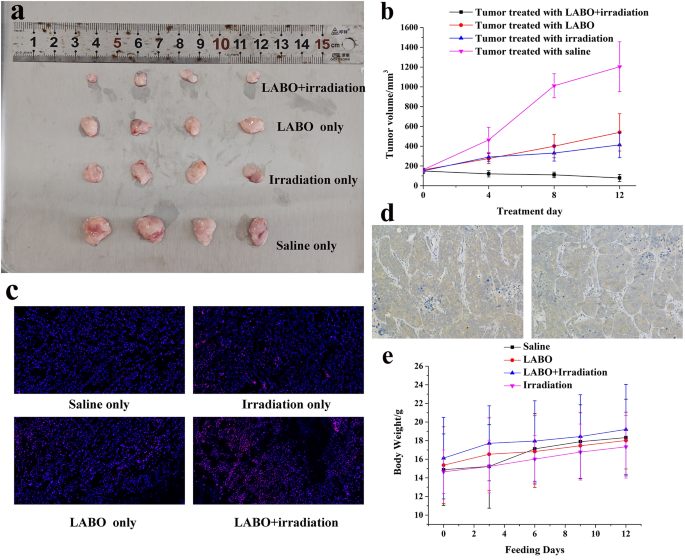
The antitumor impact of LABO NPs throughout photodynamic remedy was evaluated on the HepG2 tumor bearing nude mice. Because the leads to Fig. 6a and b demonstrated, tumors progress was considerably inhibited by LABO administration mixed with tumor irradiation throughout 12-day photodynamic remedy. Tumor volumes have been diminished from 148.8 ± 24.5 mm3 to 78.5 ± 35.2 mm3 in comparison with the controlling group, of which mice was administrated with saline and tumor volumes elevated from 161.3 ± 16.7 mm3 to 1203.4 ± 252.9 mm3. When mice was solely handled with LABO or irradiation, the antitumor results was unconspicuous, tumor enlarged throughout remedy (161.12 ± 17.88 mm3 to 539.9 ± 189.1 mm3 for LABO remedy and 150.68 ± 10.35 mm3 to 412.9 ± 128.6 mm3 for irradiation remedy).
To substantiate the era of intratumoral in-situ self-assembled nanoparticle and the photoinduced ROS, mice have been then sacrificed after remedy and tumors have been taken out for ROS staining. Because the leads to Fig. 6c demonstrated, ROS in HepG2 tumors handled with saline may very well be stained pink (left higher). In the meantime, ROS within the tumors handled with irradiation (proper higher) or LABO (left down) was elevated to some extent, however appeared to not attain the brink of tumor tolerance to exert antitumor results. Nevertheless, when tumor was handled with LABO and irradiation, important ROS outbreak within the tumor was noticed, reflecting the phototoxicity of LABO. The immunohistochemical evaluation of ASGPR was additionally carried out, as proven in Fig. 6d, to confirmed the excessive expression ASGPR on the HepG2 tumor.
To substantiate the organic safety of LABO, mouse weight was monitored throughout photodynamic remedy. Mouse in all the experimental/management teams demonstrated physique weight enhance, as proven in Fig. 6e. Physique weight of mouse handled with saline/LABO/LABO + irradiation/irradiation was initially 14.88 ± 3.84 g 15.36 ± 4.12 g, 16.12 ± 4.38 g and 14.65 ± 2.34 g respectively, and have been elevated to 17.12 ± 3.78/18.33 ± 4.12 g, 16.83 ± 3.85/18.01 ± 3.05 g, 17.96 ± 4.32/19.21 ± 4.83 g and 16.02 ± 2.55/17.34 ± 3.36 g at 6/12 days publish administration. Organ H&E staining of mice handled with LABO + irradiation was additionally carried out, as proven in Fig S11. Organs of non-target together with liver, kidney, coronary heart, spleen and lung confirmed intact construction, confirming the organic safety of LABO. Furthermore, peripheral blood samples of mice handled with LABO solely and LABO + irradiation have been additionally collected, and the biochemical index, together with erythrocyte rely, leukocyte rely, aspartate amino transferase (AST), alanine aminotransferase (ALT) and complete bilirubin (T-Bil) have been measured. Because the outcomes listed in Desk S3 indicated, all of those blood index met the traditional reference vary, reflecting the low kidney/liver damages and hematotoxicity of LABO. Photodynamic remedy was thought-about as tumor inhibiting technique with high-level safety because of the controllable irradiation space, irradiation power and irradiation time. The viscosity induced intratumoral in-situ meeting and fluorescence get well not solely assured efficient tumor inhibition, but additionally added a double assurance for the non-target safety.
Antitumor results of LABO: (a: Tumor images after photodynamic remedy, tumors from as much as down corresponded to the teams handled with LABO + irradiation, LABO, irradiation and saline; b: Tumor quantity change throughout photodynamic remedy, c: Tumor ROS staining after photodynamic remedy; d: Immunohistochemical evaluation of ASGPR expression on HepG2 tumors, e: Physique weight monitoring throughout photodynamic remedy