Synthesis and characterization of AuCePt PHNs
AuCePt PHNs have been synthesized by sacrificial galvanic alternative of Co nanoparticles within the presence of HAuCl4, Ok2PtCl6 and Ce(NO3)3. Transmission electron microscopy (TEM) confirmed that AuCePt PHNs have been spherical particles with a porous floor, hole construction and common diameter of ~ 60 nm (Fig. 1A), which is conducive to its passing by means of the hepatic sinusoid with a pore diameter of fifty–180 nm of endothelial cells to achieve hepatocytes or hepatic stellate cells [39,40,41,42]. Dynamic gentle scattering (DLS) experiments confirmed that the hydrodynamic measurement of AuCePt PHNs was ~ 66 nm (Fig. 1E). The hydrodynamic diameter measured by DLS was barely bigger than that measured by TEM, which might be assigned to the truth that the dimensions measured by DLS consisted of the diameters of each AuCePt PHNs and the hydration layer. The fundamental mapping and vitality dispersive X-ray spectroscopy (EDS) outcomes confirmed that AuCePt PHNs have been primarily composed of Au, Ce and Pt components (Fig. 1B and Fig. S1). X-ray photoelectron spectroscopy (XPS) spectra of AuCePt PHNs additionally confirmed the attribute peaks of Au, Ce and Pt components (Fig. 1C). Moreover, the high-resolution XPS spectra fitted the attribute peaks of every aspect in AuCePt PHNs (Fig. S2), which confirmed Au containing 92.62% Au0, Ce containing 28.69% Ce3+ and 71.31% Ce4+, and Pt containing 85.35% Pt0.
Excessive-resolution TEM was used to characterize the lattice spacing of the ready supplies. As proven in Fig. S3A, the lattice spacing of AuCePt PHNs contains d [111] = 0.23 nm and d [200] = 0.20 nm, indicating the excessive crystallinity of AuCePt PHNs. The X-ray diffraction sample (XRD) revealed the crystal planes of AuCePt PHNs, together with the (111), (200), (220), (311) and (222) crystal planes, equivalent to 2θ values of 38.3°, 44.7°, 64.9°, 77.7° and 81.7°, respectively (Fig. S3B). In contrast with the usual playing cards (Au//PDF # 04-0784, Ce//PDF # 31–0325 and Pt//PDF # 04-0802) and XRD sample of Au PHNs, the diffraction peaks of AuCePt PHNs have been barely shifted, revealing that the Au, Ce and Pt species fashioned a homogeneous and single-phase alloy construction [43].
The pore construction of AuCePt PHNs was analyzed by N2 adsorption experiments (Fig. 1D). The outcomes confirmed that AuCePt PHNs had two important pore sizes of three.5 nm and 6.1 nm. Furthermore, the high-resolution TEM picture additionally clearly confirmed a pore with a diameter of roughly 3.2 nm (Fig. S3A, crimson arrow), which is mutually confirmed with the outcomes of the N2 adsorption experiment.
Preparation and characterization of AuCePt PHNs-LA
As a result of LA can particularly bind asialoglycoprotein receptor (ASGPR) on the floor of the hepatocyte membrane [44], AuCePt PHNs have been modified with LA to focus on hepatocytes. L-cys with sulfhydryl and amino teams was used to hyperlink AuCePt PHNs and LA through Au-S and amide bonds. To investigate this course of, the morphological adjustments of AuCePt PHNs have been first characterised by TEM. As proven in Fig. 1E, after being modified by L-cys, the floor of AuCePt PHNs clearly confirmed a clear movie. As well as, the clear movie grew to become thicker with additional connection of LA. The DLS outcomes additionally confirmed that the diameter of AuCePt PHNs elevated with the stepwise modification of L-cys and LA. Zeta-potential outcomes (Fig. S4) confirmed that the floor potential of AuCePt PHNs decreases from ~ -15 to ~ -20 mV after which to ~ -33 mV after the modification of L-cys and LA. These outcomes confirmed the profitable grafting of L-cys and LA. A earlier examine proved that the electrostatic repulsion of nanoparticles with zeta potentials higher than 20 mV or lower than − 20 mV was adequate to take care of the steadiness of nanoparticles in solvents [45]. Thus, AuCePt PHNs-LA with an ~ -33 mV floor cost could have good stability and dispersion within the circulating system. As anticipated, the diameter of AuCePt PHNs remained steady after being saved in PBS or serum for 1 month, indicating good stability and dispersion of AuCePt PHNs underneath physiological circumstances (Fig. S5).
Subsequent, 13C nuclear magnetic resonance (13C-NMR) was used to additional affirm the profitable modification of LA on AuCePt PHNs. As a result of AuCePt PHNs didn’t comprise elemental carbon, there was no sign peak within the scanning spectrum of AuCePt PHNs (Fig. 1F). After AuCePt PHNs have been linked with L-cys and LA, sign peaks appeared at 24.7899, 25.2058, 42.6966 ~ 103.4555, 172.3389 and 176.5229 ppm. In accordance with the report [46] and the 13C-NMR scanning spectrum of L-cys and LA, the peaks at 61.0013–103.4555 ppm belong to LA, and the peaks at 24.7899, 55.895, 172.3389 ppm belong to L-cys, which confirmed that we efficiently obtained the novel AuCePt PHNs-LA compounds.
As well as, the adjustments within the practical teams of AuCePt PHNs, L-cys, and LA earlier than and after connection have been analyzed by Fourier rework infrared spectroscopy (FTIR). As proven in Fig. 1G, L-cys had S-H stretching tensile vibration (blue body) at 2550–2750 cm− 1, however the peak disappeared after L-cys mixed with AuCePt PHNs. This consequence indicated that the sulfhydryl of L-cys fashioned a extra steady Au-S bond with AuCePt PHNs [47], and AuCePt PHNs-L-cys was ready. As well as, the vibration attribute peak of C = O moved from 1741.03 cm− 1 to 1634.29 cm− 1 (crimson body), which indicated that the -COOH of LA had efficiently condensed with -NH2 to kind an amide bond [48] and proved that LA was modified on the floor of AuCePt PHNs-L-cys. Subsequently, L-cys connects AuCePt PHNs and LA by means of gold sulfur bonds and amide bonds.
Characterization of AuCePt PHNs and AuCePt PHNs-LA. (A) TEM picture of AuCePt PHNs. (B) Elemental mapping pictures of AuCePt PHNs. (C) XPS spectra of AuCePt PHNs. (D) N2 adsorption-desorption isotherms (inset) and the corresponding DFT pore measurement distribution curves of AuCePt PHNs. (E) DLS and high-magnification TEM characterization of the particle measurement and morphology of AuCePt PHNs-LA. (F) 13C-NMR spectroscopy and (G) FTIR spectroscopy of AuCePt PHNs modified by L-cys and LA
Enzyme-like exercise of AuCePt PHNs
SOD catalyzes •O2− to generate H2O2 and O2, which is the preliminary step of the scavenging ROS (Fig. 2A). To guage the SOD-like exercise of AuCePt PHNs, their efficiency in eliminating •O2− produced by xanthine (X) and xanthine oxidase (XO) response programs was monitored. The kinetic curve of absorbance at 560 nm in Fig. 2B confirmed that the absorbance rises very slowly within the presence of AuCePt PHNs. It is because AuCePt PHNs can effectively eradicate •O2− and thus inhibit the discount of NBT to blue methadone, indicating that AuCePt PHNs have wonderful SOD-like exercise. Fig. S6 indicated that AuCePt PHNs can exhibit essentially the most excellent SOD-like exercise in a weak alkali surroundings roughly at pH = 8. By evaluating the SOD-like exercise of AuCePt PHNs with the same nanozymes, AuCePt PHNs have the very best SOD-like exercise at any focus (Fig. 2C). As well as, for acquiring an in-depth understanding of the SOD-like exercise of AuCePt PHNs, its enzyme kinetic parameters have been examined. Fig. S7 revealed that the Vmax, Km, and Kcat/Km of the SOD-like exercise in AuCePt PHNs have been 2.42 × 10− 8 M·s− 1, 1.87 × 10− 5 M and 1.94 × 104 M− 1·s− 1 respectively. In contrast with the opposite three nanozymes, the SOD-like exercise of AuCePt PHNs possesses one of the best catalytic fee and the very best affinity.
H2O2 is just not solely the product of •O2− but additionally one other vital ROS. It may be catalyzed by CAT to generate O2 and H2O, which is the second key step within the ROS removing course of (Fig. 2D). Thus, the CAT-like exercise of AuCePt PHNs was evaluated by monitoring the era of O2. As proven in Fig. 2E, AuCePt PHNs may effectively catalyze H2O2 to generate O2, and exhibit the upper CAT-like exercise underneath impartial pH circumstances. The outcomes of CAT take a look at equipment additional steered that the CAT-like exercise of AuCePt PHNs is one of the best when pH = 8 (Fig. S8). Fig. S9A confirmed the O2 era fee will increase with rising AuCePt PHNs focus. The oxygen manufacturing effectivity and the residual quantity of H2O2 proved that the CAT-like exercise of AuCePt PHNs was the upper than that within the Au PHNs, AuPt PHNs or AuCe PHNs teams (Fig. S9B and Fig. 2F). As well as, the steady-state kinetic outcomes of AuCePt PHNs confirmed that Vmax, Km, and Kcat/Km of the CAT-like exercise in AuCePt PHNs have been 4.79 × 10− 6 M·s− 1, 4.26 × 10− 2 M and 90.0 M− 1·s− 1 respectively (Fig. S10), which indicated the CAT-like exercise of AuCePt PHNs possesses the higher catalytic fee and the upper affinity than that of the opposite three nanozymes.
These outcomes indicated that AuCePt PHNs current each SOD and CAT-like actions and may catalyze cascade enzyme reactions to eradicate ROS (•O2− — H2O2 — O2 + H2O). The excellent enzymatic exercise of AuCePt PHNs might be attributed to the next facets: (1) •O2− readily captures protons from water, forming HO2• and OH−. The adsorption of HO2• on the Au(111) and Pt(111) planes is a extremely exothermic course of, and the activation vitality obstacles of the Au(111) and Pt(111) planes are extraordinarily low. As soon as HO2• is adsorbed on the floor, Au and Pt exert SOD-like exercise, changing HO2• into O2 and H2O2 [49]. (2) Beneath alkaline circumstances, the Au(111) and Pt(111) planes can pre-adsorb OH, which serves because the lively website to provoke the acid-like decomposition of H2O2, and may promote the conversion of H2O2 to H2O and O2 [18]. (3) Cerium nanomaterials have a redox pair that may cycle between the + 3 and + 4 states of oxygen emptiness websites, offering wonderful SOD/CAT-like exercise [19]. Moreover, Fig. 2(C and F) confirmed our speculation that the mix of the three elements would enhance the enzyme-like exercise of the AuCePt PHNs in comparison with that of single-component or two-component nanozymes.
To research the power of AuCePt PHNs to scavenge ROS on the mobile stage, DCFH-DA was used as a fluorescent probe. As proven in Fig. 2G and Fig. S11 (A and B), therapy with AuCePt PHNs didn’t result in important adjustments in ROS ranges in regular cells. After H2O2 therapy, the fluorescence depth was considerably elevated, indicating that intracellular ROS ranges have been elevated. Nonetheless, the fluorescence was considerably diminished with the addition of AuCePt PHNs. These outcomes steered that AuCePt PHNs may scavenge exogenous ROS. To additional discover the exercise of AuCePt PHNs in scavenging endogenous ROS. Alpha mouse liver (AML-12) cells have been handled with FFAs combination to manufacture the IR mannequin, which resulted in a major enhance in ROS ranges (in contrast with regular cells). When AuCePt PHNs have been added, the ROS stage was considerably diminished, indicating that AuCePt PHNs successfully eradicated endogenous ROS.
In accordance with literature studies [26], since SOD and CAT are distributed in several organelles (Fig. S12), the manufacturing and degradation of H2O2 underneath pure circumstances should be transported between organelles. Nonetheless, AuCePt PHNs have wonderful SOD- and CAT-like actions concurrently, which might catalyze cascade reactions, thus saving the transport means of H2O2 between organelles. It’s thus clear that the cascade response exercise of AuCePt PHNs to ROS could also be very useful to enhance the scavenging effectivity of ROS.
Characterization of the SOD and CAT-like actions of AuCePt PHNs. (A) Simulation diagram for the SOD-like exercise of AuCePt PHNs. (B) Typical kinetic curves of A − A0 (560 nm) for monitoring the discount of NBT with •O2−. (C) •O2− elimination effectivity of comparable nanozymes with completely different concentrations. (D) Simulation diagram for the CAT-like exercise of AuCePt PHNs. (E) Typical kinetic curves of AuCePt PHNs-mediated O2 era from H2O2 at completely different pH values. (F) H2O2 decomposition effectivity of comparable nanozymes with completely different concentrations. (G) Results of AuCePt PHNs on ROS ranges in AML-12 cells handled with H2O2 or FFAs
Loading capability and launch effectivity of AuCePt PHNs for DSF
Within the above experiments, it has been confirmed that AuCePt PHNs have a porous and hole bodily construction. The literature has reported that this particular bodily construction endows nanoparticles with a robust load capability [50]. In accordance with the becoming linear equations of DSF detected by high-performance liquid chromatography (HPLC) (Fig. S13) and the calculation equation in Sect. 2.8, the loading fee of AuCePt PHNs for DSF was 37.3% ± 2.9, and the entrapment fee was 47.8% ± 5.8.
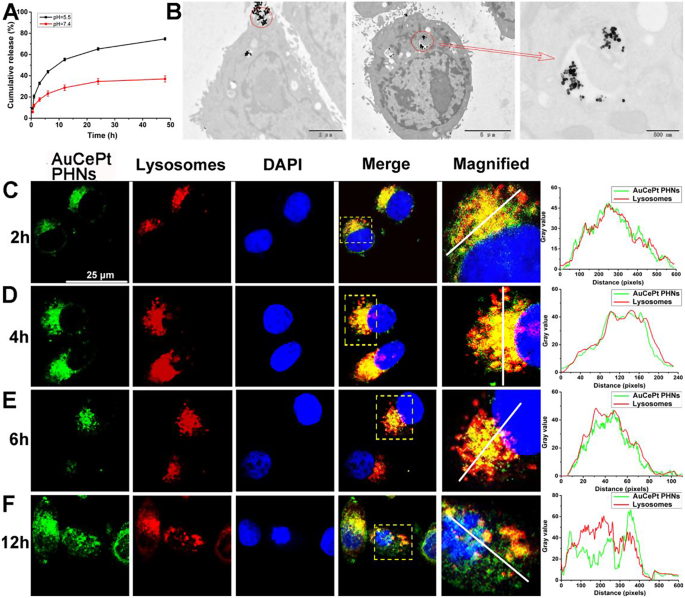
Subsequent, to research the discharge effectivity of AuCePt PHNs for DSF at completely different pH values, the cumulative launch fee of DSF in 0–48 h was measured. Determine 3A confirmed that the discharge of DSF from AuCePt PHNs is principally concentrated inside 24 h and has a major pH dependence. When the pH of the dissolution medium was 7.4, the discharge effectivity in 48 h was solely 36.98%, whereas when the pH was 5.5, the discharge effectivity reached roughly 74.76%. The pH dependence can cut back the nonspecific launch of AuCePt PHNs to medication in peripheral blood (pH = 7.35–7.45), whereas some organelles with decrease pH (equivalent to lysosomes) can promote the discharge of DSF from AuCePt PHNs [51].
Cytotoxicity and intracellular distribution of AuCePt PHNs
To research the cytotoxicity of AuCePt PHNs and DSF, we first used Cell Counting Package-8 (CCK-8) in AML-12 cells (Fig. S14A and B). The outcomes confirmed no important cytotoxicity when the concentrations of AuCePt PHNs and DSF have been lower than 100 µg·mL− 1 and eight µM, respectively. As well as, movement cytometric evaluation additional confirmed that there was no important proof of cell apoptosis in AML-12 cells after therapy with 100 µg·mL− 1 AuCePt PHNs or 8 µM DSF (Fig. S15A and B). Determine 3B clearly confirmed that AuCePt PHNs-LA was engulfed by cells by means of endocytosis and was encapsulated in endosomes. Importantly, the membrane and nuclear constructions of the AuCePt PHNs-LA-treated AML-12 cells have been clear and full, which additionally proved that AuCePt PHNs-LA has good biocompatibility. As well as, we discovered that after AuCePt PHNs-LA was endocytosed, these endocytic particles have been uniformly dispersed, which successfully averted aggregation-induced cytotoxicity.
To check the intracellular distribution of AuCePt PHNs-LA, AML-12 cells have been handled with LysoTracker (crimson) and AuCePt PHNs-LA loaded with coumarin 6 (AuCePt PHNs-LA@cou6, inexperienced), and fluorescence colocalization evaluation was carried out at completely different time factors. The outcomes confirmed that the longer the therapy time of AuCePt PHNs-LA@cou6 was, the bigger the yellow space (colocalization space) in cells, which peaked at 6 h (Fig. 3C-F). This phenomenon indicated that the quantity of AuCePt PHNs-LA coming into the lysosome was the most important after incubation for six h. Nonetheless, when the cells have been incubated for 12 h, the inexperienced fluorescence and crimson fluorescence have been clearly separated, indicating that some AuCePt PHNs-LA had escaped from the lysosome. The outcomes of intracellular distribution and fluorescence colocalization indicated that AuCePt PHNs-LA may enter the lysosome in order that DSF was successfully launched within the acidic surroundings and will escape from the lysosome to play a therapeutic position within the cytoplasm.
Bodily properties and intracellular localization of AuCePt PHNs. (A) Effectivity-time curves of AuCePt PHNs for DSF launch at completely different pH values. (B) TEM pictures of the intracellular distribution of AuCePt PHNs-LA. (C–F) Colocalization evaluation for AuCePt PHNs (inexperienced) and lysosomes (crimson) at completely different time durations
Results of AuCePt PHNs-LA@DSF on intracellular IR
The researches of Prof. Mailloux and Prof. Solar proved that DSF can clear ROS and cut back OS [52, 53]. Moreover, the above experiments confirmed that AuCePt PHNs-LA can cut back the endogenous and exogenous ROS. Subsequently, we speculated that AuCePt PHNs-LA and DSF can collectively enhance IR by lowering OS. To discover the impact of AuCePt PHNs-LA@DSF on cell-level IR, we carried out a 2-NBDG uptake experiment in AML-12 IR cells. Cell fluorescence imaging confirmed that the fluorescence depth of the 4 therapy teams (DSF, AuCePt PHNs-LA, and AuCePt PHNs-LA@DSF) was larger than that of management group (Fig. 4A). This discovering steered that they will enhance glucose uptake in IR cells. Amongst them, AuCePt PHNs-LA@DSF group had the very best glucose uptake fee. Related outcomes have been obtained by movement cytometry evaluation (Fig. 4B). As well as, the glycogen staining outcomes confirmed that the glycogen content material in IR cells handled with AuCePt PHNs-LA and DSF was considerably elevated, whereas the cell glycogen content material was the very best after AuCePt PHNs-LA@DSF therapy (Fig. 4C and D). Subsequently, the AuCePt PHNs-LA@DSF can effectively promote glucose uptake and glycogen synthesis in IR cells, thereby enhancing glucose metabolism.
Affect of AuCePt PHNs-LA@DSF on gluconeogenesis and insulin signaling molecules in IR hepatocytes
It has been properly documented that OS promote the incidence and growth of IR by means of insulin signaling pathway and gluconeogenesis signaling pathway [43, 44]. Subsequently, we subsequent explored the affect of AuCePt PHNs-LA@DSF on the phosphorylation and expression of key molecules in these two traditional pathways, together with IRS-1, AKT (a serine/threonine kinase and a flexible node in insulin sign transduction) [54], FOXO-1 (an vital substrate molecule downstream of IRS-1/AKT, mediating gluconeogenesis) [55], and PEPCK (a rate-limiting enzyme within the gluconeogenesis pathway) [56]. Firstly, we investigated the consequences of various concentrations of Au and DSF on AKT phosphorylation. As proven in Fig. 4E-H, AuCePt PHNs-LA or DSF improved AKT phosphorylation ranges in IR AML-12 cells in a dose-dependent method. Secondly, by evaluating the consequences of various nanomaterials on the AKT phosphorylation stage, it could possibly be seen that AuCePt PHNs had the very best regulation effectivity (Fig. S16). Considerably, therapy with DSF, AuCePt PHNs-LA, AuCePt PHNs-LA@DSF resulted in elevated IRS-1 and AKT phosphorylation ranges, and decreased FOXO1 and PEPCK expression in IR AML-12 cells. Particularly, the impact of AuCePt PHNs-LA@DSF is one of the best (Fig. 4I ~ M). These outcomes indicated that AuCePt PHNs-LA@DSF can enhance IR by regulating the insulin signaling pathway and the gluconeogenesis signaling pathway.
Results of AuCePt PHNs-LA@DSF on glucose metabolism and the expression of insulin signaling molecules in vitro. The management group is the IR cells mannequin, which is ready by AML-12 cells incubating with FFAs for 20 h. (A) Fluorescent pictures for 2-NBDG uptake stage. (B) Circulate cytometry for 2-NBDG uptake stage. (C) PAS staining for glycogen contents. (D) Quantitative evaluation of glycogen contents. WB experiments: (E ~ F) Akt phosphorylation stage in AuCePt PHNs-treated AML-12 IR cells. (G ~ H) Akt phosphorylation stage in DSF-treated AML-12 IR cells. (I ~ M) The expression and phosphorylation of key proteins in insulin signaling pathway and gluconeogenesis signaling pathway in DSF, AuCePt PHNs-LA, and AuCePt PHNs-LA@DSF-treated AML-12 IR cells. Knowledge are expressed because the imply ± commonplace deviation (n = 3 unbiased experiments). *p < 0.05, **p < 0.01, and ***p < 0.001 vs. management
Focusing on effectivity, biodistribution and toxicity of AuCePt PHNs-LA in vivo
To research whether or not the LA-modified AuCePt PHNs can promote the phagocytosis of hepatocytes, we used doxorubicin (DOX) to label AuCePt PHNs and AuCePt PHNs-LA to assemble fluorescent probes. AML-12 or HEK293T cells have been handled with AuCePt PHNs or AuCePt PHNs-LA as indicated within the Strategies. We discovered that the fluorescence sign in AML-12 cells handled with AuCePt PHNs-LA@DOX was considerably larger than that in AuCePt PHNs@DOX-treated AML-12 cells, indicating that LA modification improved the phagocytosis effectivity of AuCePt PHNs in AML-12 cells (Fig. 5A). As well as, in AuCePt PHNs-LA@DOX-treated cells, the fluorescence sign in HEK293T cells was considerably weaker than that in AML-12 cells, indicating that LA modification promoted the ASGPR-mediated endocytosis of AuCePt PHNs-LA, which is extremely expressed on the floor of hepatocytes. Primarily based on LA practical modification and the pH dependence of drug launch, AuCePt PHNs-LA may effectively goal the liver to launch DSF, thereby lowering the nonspecific distribution and toxicity of nanomaterials and medicines.
To grasp the biodistribution of AuCePt PHNs-LA in vivo, WT mice have been injected with Cy7.5-labeled AuCePt PHNs or AuCePt PHNs-LA through the tail vein. In vivo fluorescence imaging confirmed that AuCePt PHNs-LA@Cy7.5 amassed extra within the liver than did AuCePt PHNs@Cy7.5 after 3 h of injection, suggesting that AuCePt PHNs-LA have been extra focused (Fig. 5B). Constantly, organ fluorescence imaging additionally confirmed this conclusion (Fig. 5C and Fig. S17). We continued to discover the distribution of AuCePt PHNs-LA in organs at completely different time factors. The fluorescence of the liver decreased progressively over time. Importantly, the liver nonetheless retained a robust fluorescence sign after 48 h, which indicated that AuCePt PHNs-LA can keep within the liver for a very long time to exert its enzyme-like exercise. The fluorescence depth within the kidney elevated, suggesting that the fabric could also be excreted by means of the kidney.
Subsequent, to confirm the toxicity of DSF and AuCePt PHNs-LA in vivo, we analyzed routine blood and biochemical indicators in mice. In contrast with HFD group, DSF, AuCePt PHNs-LA, and AuCePt PHNs-LA@DSF therapy didn’t considerably have an effect on varied indicators within the mice (Desk S1). As well as, there was no important change in H&E staining of assorted tissues or organs (Fig. S18). These outcomes indicated that AuCePt PHNs-LA@DSF didn’t trigger cardiac or renal dysfunction or systemic practical problems in mice. Subsequently, AuCePt PHNs-LA@DSF has good biosecurity.
Impact of AuCePt PHNs-LA@DSF on vitality expenditure in vivo
To ascertain HFD-induced overweight animal fashions, WT mice have been fed a HFD for 12 weeks and handled with DSF, AuCePt PHNs-LA, or AuCePt PHNs-LA@DSF for 16 days (Fig. 5D). In contrast with the HFD group, the mice within the therapy teams have been considerably smaller physique weight and in measurement (Fig. 5E and F). Particularly, the burden loss was most blatant within the AuCePt PHNs-LA@DSF-treated group. As well as, the morphological, H&E staining and oil-red O staining pictures of the liver confirmed that lipid deposition was considerably diminished within the therapy teams (Fig. 5G and Fig. S19 ~ 20). Equally, amongst a number of therapies, the AuCePt PHNs-LA@DSF therapy was the simplest at lowering lipid deposition.
One of many vital causes for weight problems is an imbalance in vitality metabolism, that’s, a rise in vitality storage and a lower in vitality consumption. Subsequently, we measured the vitality expenditure in every therapy group with metabolic cages. We discovered that oxygen consumption (VO2) (Fig. 5H) and carbon dioxide (VCO2) manufacturing (Fig. S21A and B) elevated considerably within the three therapy teams, indicating the next metabolic fee. The respiratory alternate ratio (RER) additionally elevated to various levels, particularly within the AuCePt PHNsPHN-LA@DSF group (Fig. 5I). The next RER meant the next utilization fee of carbohydrates, implying that IR was alleviated. Furthermore, the whole-body vitality expenditure and rectal temperature additionally elevated considerably (Fig. S21C and D). General, these knowledge indicated that AuCePt PHNs-LA@DSF therapy considerably diminished physique weight and liver lipid accumulation and elevated general vitality consumption. Furthermore, our knowledge steered that AuCePt PHNs-LA and DSF exhibit mixed therapeutic results. As well as, these outcomes proved that the rise in vitality consumption is the principle reason behind weight reduction within the AuCePt PHNs-LA@DSF therapy group.
Validation of AuCePt PHNs-LA for focusing on the liver: (A) Fluorescence emission spectroscopy and quantitative evaluation of the endocytosis stage of AuCePt PHNs by completely different cells earlier than and after LA modification (B) In vivo imaging exhibiting the distribution of AuCePt PHNs or AuCePt PHNs-LA in mice after tail vein injection. (C) Fluorescence pictures exhibiting the distribution of AuCePt PHNs and AuCePt PHNs-LA in several organs after tail vein injection. Results of AuCePt PHNs-LA@DSF on physique weight and vitality metabolism in HFD-fed C57BL/6J mice: (D) Experimental technique for measuring vitality expenditure. Cumulative physique weight (E), consultant pictures of the entire physique (F), and HE staining of liver tissue sections (G) of mice subjected to completely different therapies (a. HFD, b. DSF, c. AuCePt PHNs-LA, and d. AuCePt PHNs-LA@DSF). (H) 24 h oxygen consumption. (I) Respiratory alternate ratio (RER: VCO2/VO2). Knowledge are expressed because the imply ± commonplace deviation or commonplace error of imply (n = 4 ~ 6 mice for every group), * p < 0.05, **p < 0.01, ***p < 0.001 vs. HFD group
Results of AuCePt PHNs-LA@DSF on glucose metabolism and IR in vivo
To guage the impact of AuCePt PHNs-LA@DSF on insulin sensitivity in vivo, WT mice have been fed a HFD for 12 weeks to assemble an IR mannequin after which handled with DSF, AuCePt PHNs-LA, or AuCePt PHNs-LA@DSF for 16 days through the tail vein (Fig. 6A). As proven in Fig. 6B ~ C, fasting blood glucose and insulin ranges within the three therapy teams have been markedly diminished. Particularly, the fasting blood glucose and insulin ranges of AuCePt PHNs-LA@DSF group mice have been very near these of the management group mice. Furthermore, through the GTT and ITT experiments, the blood glucose stage and the realm underneath the glucose curve (AUC) at every time level within the three therapy teams considerably decreased, whereas these within the AuCePt PHNs-LA@DSF-treated group have been the bottom (Fig. 6D ~ G).
To additional discover the impact of AuCePt PHNs-LA@DSF on insulin sensitivity within the liver on the molecular stage, we measured the extent of phosphorylation or expression of insulin and gluconeogenesis signaling molecules extracted from liver tissues. In contrast with the management group, the phosphorylation ranges of IRS-1 and AKT have been considerably elevated within the liver, whereas the degrees of PEPCK and FOXO1 proteins have been considerably decreased, suggesting that insulin sensitivity was improved within the liver, particularly in AuCePt PHNs-LA@DSF-treated mice (Fig. 6H ~ L).
Continual low-grade irritation results in liver steatosis, IR and weight problems. Thus, the impact of AuCePt PHNs-LA@DSF on OS and irritation within the liver was analyzed. The ROS-scavenging effectivity of every therapy group was measured utilizing DCFH-DA because the fluorescent probe. We discovered that AuCePt PHNs-LA and DSF therapy considerably diminished ROS ranges within the livers of HFD-fed C57BL/6J mice (Fig. S22). The fluorescence depth of the AuCePt PHNs-LA@DSF-treated group was the weakest, indicating that its antioxidant capability was the strongest. As well as, we investigated the consequences of AuCePt PHNs-LA@DSF on inflammatory elements and liver enzymes in vivo. As proven in Fig. 6M ~ P, these three therapy teams all exhibited important decreases in serum TNF-α and IL-6 ranges and important enhancements in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ranges. These adjustments have been most blatant within the AuCePt PHNs-LA@DSF-treated group, suggesting that the mix of DSF and AuCePt PHNs-LA had the strongest anti-inflammatory impact and ameliorated liver damage.
Results of AuCePt PHNs-LA@DSF on glucose metabolism and insulin sensitivity in HFD-fed mice. (A) Experimental technique for GTT and ITT. (B) Fasting blood glucose. (C) Fasting insulin. (D) and (E) Blood glucose and AUCGTT through the GTT. (F) and (G) Blood glucose and AUCITT through the ITT. (H – L) Protein expression of PEPCK and FOXO1 and phosphorylation ranges of AKT and IRS-1. (M) Serum TNF-α ranges. (N) Serum IL-6 ranges. (O) Serum AST ranges. (P) Serum ALT ranges. Knowledge are expressed because the imply ± commonplace deviation (n = 6 mice for every group), * p < 0.05, **p < 0.01 and ***p < 0.001 vs. management
Efficacy verification of AuCePt PHNs-LA@DSF on ob/ob mice
Ob/ob mice is a traditional mannequin of genetic weight problems, accompanied by liver steatosis [57]. Subsequently, to guage the consequences of AuCePt PHNs-LA@DSF therapy on IR, weight problems and fatty liver, ob/ob mice have been handled with AuCePt PHNs-LA@DSF for 16 days through the tail vein (Fig. 7A). The outcomes confirmed that AuCePt PHNs-LA@DSF therapy considerably diminished physique weight (Fig. 7B ~ C, and Fig. S23A), fasting blood glucose (Fig. 7D), fasting insulin (Fig. S23B), and liver lipid deposition (Fig. 7E and Fig. S23C). As well as, the glucose tolerance and insulin tolerance improved considerably (Fig. 7F ~ I). Furthermore, ROS ranges and irritation within the livers of ob/ob mice have been alleviated (Fig. S23D). Western blot evaluation revealed that in contrast with the SD-fed management group, the protein expression of PEPCK and FOXO1 in AuCePt PHNs-LA@DSF-treated ob/ob mice was considerably inhibited, whereas the phosphorylation of IRS-1 and AKT have been considerably elevated (Fig. 7J-N). These knowledge indicated that AuCePt PHNs-LA@DSF therapy effectively inhibited gluconeogenesis and improved insulin sensitivity in ob/ob mice.
Results of AuCePt PHNs-LA@DSF on glucose metabolism and insulin sensitivity in ob/ob mice. (A) Schematic illustration of the experimental process. (B) Consultant pictures of the entire physique of the mice. (C) Physique weight. (D) Fasting blood glucose. (E) Hepatic H&E staining. (F) and (G) Blood glucose and AUCGTT through the GTT. (H) and (I) Blood glucose and AUCITT through the ITT. (J ~ N) Expression of PEPCK and FOXO1 proteins and phosphorylation ranges of AKT and IRS-1. Knowledge are expressed because the imply ± commonplace deviation (n = 4 ~ 6 mice for every group), **p < 0.01, ***p < 0.001