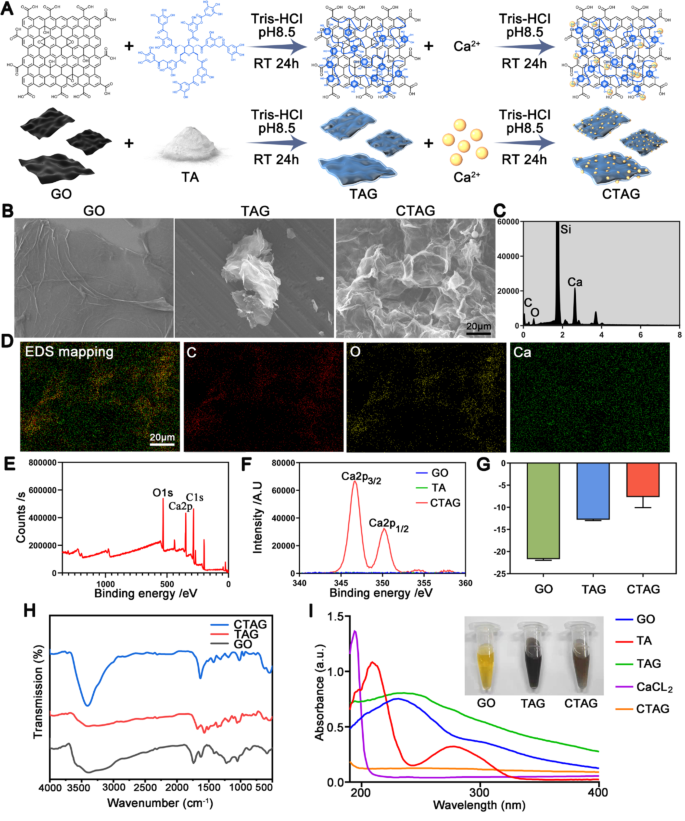
Characterization of CTAG
On this research, CTAG was ready by chemical chelation utilizing GO, TA, and calcium chloride as uncooked supplies (Fig. 2A). The SEM pictures present the classical paper-sheet layered construction of GO (Fig. 2B), which is a nanosheet with a uniform thickness of roughly 1.1 nm (Fig. S1A, B). The agglomeration of TAG into clusters was noticed with TA as a lowering agent adsorbed onto the graphene nanosheets, and the thickness elevated to roughly 2.5 nm as measured by AFM (Fig. S1A, B). After chelation with Ca2+ once more, the floor construction was not distinctly completely different, though the thickness barely elevated (3.1 nm). In the meantime, SEM-EDS proved that calcium was uniformly current on the floor of CTAG flakes (Fig. 2C, D; Si: substrate), indicating that Ca2+ ions have been efficiently certain to TA. The TEM pictures of GO, TAG, and CTAG (Fig. S1C) confirmed no vital variations in morphology, and all demonstrated a silk-like look.
The XPS outcomes (Fig. 2E) demonstrated that CTAG comprises a number of calcium teams along with carbon and oxygen teams in contrast with GO. The high-resolution Ca 2p XPS spectrum of CTAG (Fig. 2F) confirmed two separate peaks at 346.68 mV and 350.18 mV belonging to the binding energies of Ca 2p3/2 and Ca 2p1/2 of Ca, respectively. Furthermore, the zeta potential of CTAG elevated from − 21 mV at GO to -7 mV owing to the addition of Ca2+ and TA (Fig. 2G). FTIR spectral evaluation confirmed that GO displayed a number of peaks within the 800–1800 cm− 1 vary, which may be assigned to numerous purposeful teams, resembling 1050 cm− 1, 1223 cm− 1, 1419 cm− 1, and 1740 cm− 1, equivalent to the C-O-C stretching vibration, C-OH stretching vibration, C-OH bending vibration, and ester-based carbon-oxygen double bond, respectively (Fig. 2H). After discount by TA, the TAG confirmed peaks of TA at 1571 cm− 1 and 1511 cm− 1 equivalent to the stretching vibration of the benzene ring carbon-carbon double bond, which can be owing to the massive quantity of TA masking the infrared alerts of graphene. It’s also potential that with the discount of GO, the height of the oxygen purposeful group regularly decreases and even disappears fully [43], primarily manifested as the height of TA. After including calcium chloride to the blended samples, the absorption on the carbonyl group, which might coordinate, modified from the attribute peak at 1680 cm− 1 to 1634 cm− 1. The peaks of the benzoyl ring have been superimposed, in order that stronger coordination with Ca2+ than that of the opposite teams was presumed.
The traditional UV spectra of visible-light GO confirmed two attribute peaks at roughly 230 and 300 nm, equivalent to fragrant C-C and C = O bonds, respectively (Fig. 2I). After the addition of TA, the height at 230 nm within the TAG spectrum shifted to 255 nm. The height at 300 nm disappeared, indicating that GO discount by TA was profitable [37]. In the meantime, the weak peak of TAG at roughly 280 nm could also be owing to the n-π* transition of floor TA [44]. The CTAG peak tends to be usually steady, presumably as a result of protection of Ca2+ ions, which affected the detection of the attribute peaks. Moreover, we took macroscopic pictures of GO, TAG, and CTAG dissolved in water (Fig. 2I), whereby GO diminished by TA was black, according to earlier research [45]. Right here, the brownish coloration of CTAG was most likely owing to Ca2+ protection, which modified the pure black state. In abstract, these outcomes collectively recommend that CTAG has been efficiently synthesized via steel chelation and has the potential to be biologically energetic in the long run.
Moreover, we investigated the endocytosis of CTAG by BMSCs. R6G-labeled CTAG was ready and photographed (Fig. S2A). BMSCs co-cultured with CTAG have been initially noticed below a microscope to localize the cells that had endocytosed CTAG, as indicated by the arrows (Fig. S2B). Then, CTAG endocytosis by BMSCs was confirmed via a collection of CLSM part photographs alongside the Z-axis (Fig. S2C). The outcomes additional indicated that CTAG has good biocompatibility.
Preparation and characterization of CTAG. (A) Schematic diagram of CTAG synthesis. (B) SEM pictures of GO, TAG, and CTAG. (C) The SEM vitality spectrum of CTAG. (D) Elemental mapping of C, O, and Ca of CTAG. (E, F) XPS evaluation of CTAG surfaces. (G) Zeta potential of GO, TAG, and CTAG. (H) FTIR spectra of GO, TAG, and CTAG. (I) UV–Vis spectra of GO, TA, TAG, CaCl2, and CTAG
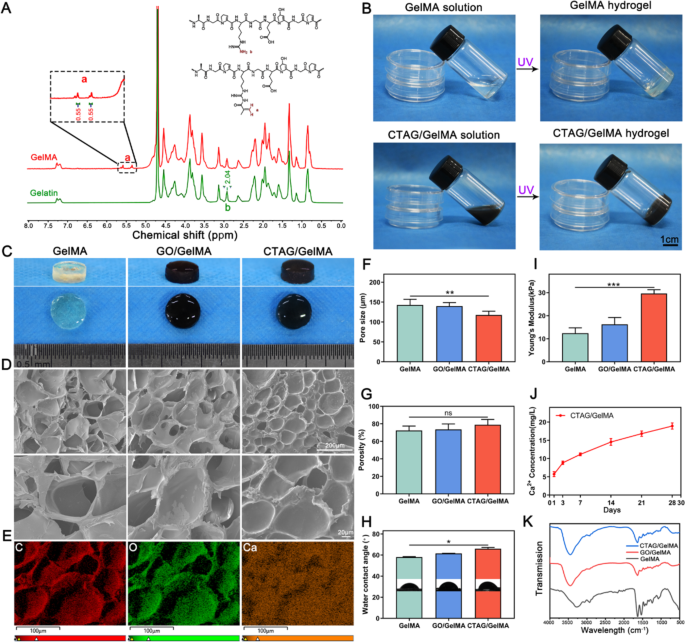
Characterization of the CTAG/GelMA hydrogel scaffolds
GelMA is a modified type of gelatin with MA, and the grafting charge of GelMA was decided by 1H-NMR spectroscopy (Fig. 3A). The alkene-hydrogen peak of acrylamide was noticed within the GelMA (5.5 ppm) spectra, and the substitution ratio was calculated to be 35.03% by integrating the height space in 1H-NMR. The CTAG/GelMA composite hydrogels have been ready by merely mixing CTAG with GelMA, and the mixtures have been gelated, with a black look (Fig. 3B, C). The SEM pictures exhibited that each one teams of hydrogels contained extremely porous microstructures after lyophilization (Fig. 3D). The pore partitions of the CTAG/GelMA hydrogels have been clean, indicating that the CTAG particles have been uniformly dispersed, and encapsulated contained in the GelMA hydrogels, which is according to the distribution of Ca2+ as detected by SEM-EDS elemental mapping (Fig. 3E). Moreover, the incorporation of CTAG didn’t affect GelMA hydrogel porosity considerably, and the pore dimension was solely decreased barely (Fig. 3F and G). Subsequently, we discovered that including CTAG doesn’t enhance the hydrophilicity of the GelMA hydrogel, and the hydrophilicity decreases (Fig. 3H). Nonetheless, the addition of CTAG significantly enhanced the mechanical properties of the GelMA hydrogels, which have been extra favorable for the therapeutic of bone defects than the opposite hydrogels (Fig. 3I).
Subsequently, slow-release options of the CTAG/GelMA hydrogels demonstrated that Ca2+ ions may be launched slowly and repeatedly over one month (Fig. 3J). The outcomes recommend that the Ca2+ launch from CTAG/GelMA hydrogels in vivo may be sustained till their fundamental degradation (4 weeks, Fig. S9) and by no means reaches a peak as a result of low launch. Such sluggish and minimal launch is important as a result of excessive calcium concentrations can result in intracellular calcium overload, leading to apoptosis [46]. As well as, low launch constantly promotes Ca2+ movement from the surface of the cell physique to the within (Fig. S3), enhancing its osteogenic results. Lastly, FTIR spectroscopy outcomes confirmed that the curve patterns of every group of hydrogels have been primarily attribute GelMA peaks, with just a few slight variations in depth at particular areas (Fig. 3Ok). The outcomes are according to earlier findings that the unique nature of the matrix, dimension, morphology, and content material of the integrated supplies, in addition to the filler-matrix interplay, affect the properties of the composites [47]. Conclusively, all of the above experiments demonstrated that CTAG/GelMA hydrogel has good mechanical properties and biosafety and might obtain sluggish Ca2+ launch throughout bone regeneration.
Characterization of CTAG/GelMA hydrogels. (A) 1H NMR spectra of gelatin and GelMA. (B) Images of the method of gelation of hydrogels. (C) Gross look of composite hydrogels after gelation. (D) SEM pictures of composite hydrogels. (E) EDS elemental mapping for CTAG/GelMA hydrogels. (F, G) Pore dimension and porosity of composite hydrogels. (H) WCA of composite hydrogels. (I) Younger’s modulus of composite hydrogels. (J) Launch curves of Ca2+ of CTAG/GelMA hydrogels. (Ok) FTIR spectra of composite hydrogels. (NS, no vital distinction; *, P < 0.05; **, P < 0.01; ***, P < 0.001)
Cytocompatibility analysis of the CTAG/GelMA hydrogel in vitro
To find out the optimum focus of CTAG added to the GelMA hydrogel, we used completely different concentrations of CTAG/GelMA hydrogel (0, 1, 2, 4, 6, and eight mg/mL) to incubate the cells. We examined the variations of their proliferative and osteogenic results (Fig. S4). Amongst them, 4 mg/mL CTAG had probably the most obvious proliferative impact on BMSCs. Cell development exercise decreased when the focus was additional elevated to six and eight mg/mL, which indicated that an excessively excessive CTAG focus would have a sure poisonous impact on the cells (Fig. S4A, B and S4E, F). ALP and ARS staining additionally confirmed that the osteogenic impact of 4 mg/mL CTAG was probably the most vital in comparison with the management (0 mg/mL) (Fig. S4C, D and S4G, H). Moreover, we examined {the electrical} conductivity of GelMA hydrogels with completely different CTAG concentrations (Fig. S5). The conductivity at 0 mg/mL originates from the residual carboxyl and amino teams of the gelatin chains. Subsequently, the gradual improve in conductivity with the addition of CTAG is especially attributed to the conductivity of GO. CTAG/GelMA (4 mg/mL) has a conductivity of roughly 8 × 10− 5 S/cm, simply throughout the conductivity vary appropriate for bone [48]. Mixed with the earlier materials characterization outcomes, the addition of 4 mg/mL CTAG was useful for the mechanical energy of the GelMA hydrogel (Fig. 3I). Bone progenitor cells are delicate to mechanical alerts and lack of biophysical forces within the pure atmosphere have an effect on the formation, resorption, and adaptation of bone tissue considerably [49]. Due to this fact, enhancing the mechanical energy of the hydrogel can enhance the osteogenic differentiation skill of MSCs. As well as, the porosity and pore dimension of the hydrogel are key elements to think about when assessing the efficacy of biomaterials as a result of they’ll have an effect on nutrient and oxygen transport [50]. Based mostly on the outcomes, 4 mg/mL CTAG supplementation had solely a minimal impact on the porosity and pore dimension of GelMA (Fig. 3F, G), which didn’t have an effect on the biocompatibility of the CTAG/GelMA hydrogel (Fig. S4A, B). Due to this fact, based mostly on the biocompatibility, osteogenesis, and electrical conductivity outcomes, we lastly chosen 4 mg/mL because the uniform experimental focus.
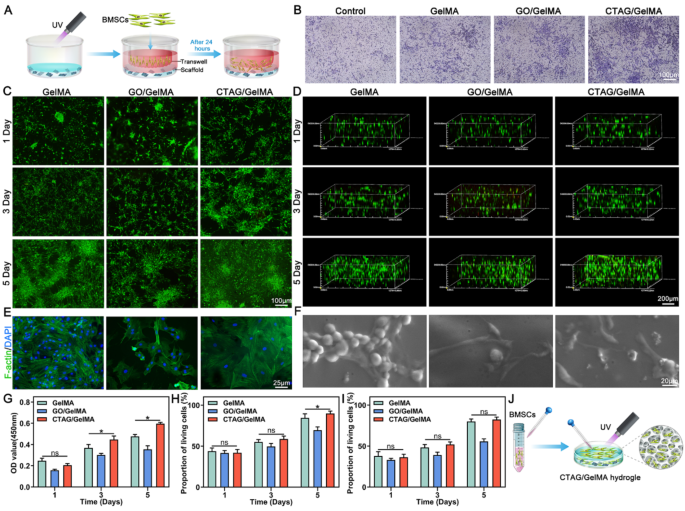
The professional-migratory impact of the CTAG/GelMA hydrogels on BMSCs was explored utilizing Transwell migration assay (Fig. 4A); the staining outcomes confirmed that the migratory performances of BMSCs by the CTAG/GelMA hydrogel have been a lot better than these of the opposite teams, which has a excessive software worth for selling bone therapeutic (Fig. 4B). Subsequently, we examined the expansion of BMSCs on the floor and within the hydrogel respectively. When rising on the floor (Fig. 4C, H), CTAG/GelMA group cells proliferated considerably extra quickly and will develop everywhere in the floor on day 5. Whereas rising within the 3D community of the hydrogel (Fig. 4D, I), the cells exhibited some penetration capabilities. They may develop uniformly all through all hydrogel areas, as proven in Fig. 4J. The CCK-8 outcomes additional quantitatively verified the cell proliferation exercise between teams (Fig. 4G), and quantitative statistical analyses demonstrated the wonderful biocompatibility of the CTAG/GelMA hydrogel. Notably, the GO/GelMA group inhibited BMSC development, and the expansion charge was diminished considerably, which can be associated to GO focus. When the GO content material exceeds a sure degree, it induces cytotoxicity and impacts cell survival and development [51, 52]. Within the current research, our outcomes demonstrated that GO at a focus of 4 mg/mL had a major poisonous impact on cells. Nevertheless, chemical chelation of GO with TA and Ca2+ to kind CTAG had no apparent poisonous impact on BMSCs, and even promoted cell proliferation, indicating that CTAG diminished the destructive impact of GO on BMSCs.
The outcomes of cytoskeleton staining confirmed that the cells on the CTAG/GelMA hydrogel unfold higher and adhered extra tightly to the substrate (Fig. 4E). In the meantime, the ESEM imaging confirmed that the GelMA group contained solely a tiny variety of pseudopods, most of which have been clustered collectively in a spherical form and didn’t wholly unfold on the hydrogel. In distinction, the cells adhering to the GO/GelMA and CTAG/GelMA hydrogels exhibited a better-stretching morphology, extending many branched filamentous pseudopods. Specifically, the CTAG/GelMA hydrogel cells have been properly distributed with typical elongated spindles and osteoblast-like morphology, indicating their good development standing. This can be attributed to its appropriate mechanical energy and the motion of CTAG, the place non-covalent interactions improve the adsorption of ECM proteins on the rGO floor [53]. Latest research verify that cell morphology performs a vital function in regulating the phenotype of cells, and an elongated spindle-shaped morphology is carefully associated to the adhesion, proliferation, and differentiation of osteoblast-associated cells [54]. Apart from, CTAG/GelMA hydrogel can adhere to the cranium and may be fastened within the faulty a part of the cranium (Fig. S6). That is primarily as a result of polyphenol moiety in TA, which reveals a powerful binding affinity to the thiol and amino teams of proteins on the tissue floor [55]. Altogether, CTAG/GelMA hydrogels may facilitate the migration, proliferation, and adhesion of BMSCs with good cytocompatibility.
Cytocompatibility of composite hydrogels. (A) Schematic illustration of the migration means of BMSCs by composite hydrogels. (B) Crystal violet-stained migrating cells in Transwell migration assays. (C) Stay/useless assay of BMSCs on hydrogel surfaces. (D) Stay/useless assay of 3D cultured BMSCs. (E) Cytoskeleton staining. (F) ESEM pictures of BMSCs. (G) CCK-8 evaluation. (H) Quantification of cell reside/useless assay on hydrogel surfaces. (I) Quantifying reside/useless assay of 3D cultured BMSCs. (J) Schematic diagram of BMSCs cultured on the floor and within the hydrogel. (NS, no vital distinction; *, P < 0.05)
Analysis of the CTAG/GelMA hydrogel for bone differentiation in vitro
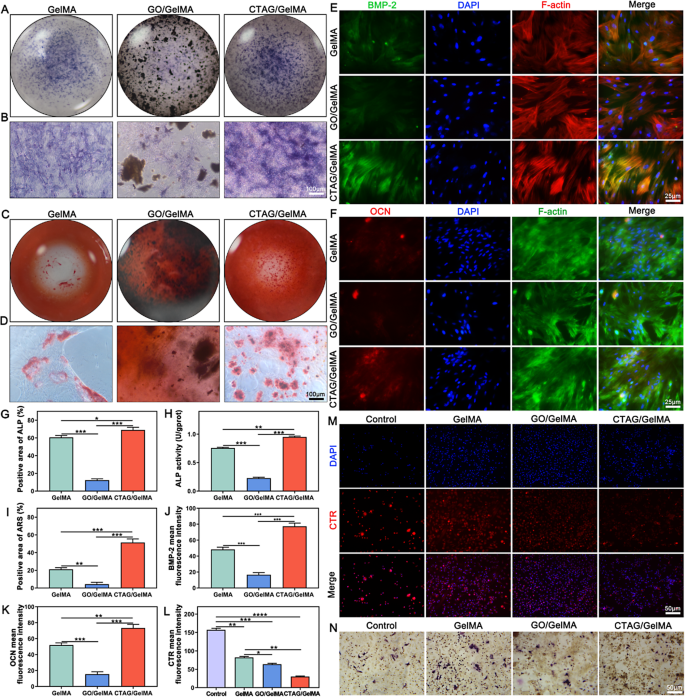
ALP staining was used to find out the results of the CTAG/GelMA hydrogel on early osteogenic differentiation. Each macroscopic (Fig. 5A) and native zoomed-in pictures (Fig. 5B) clearly present that the CTAG/GelMA group had probably the most ALP-positive cells and probably the most profound staining. Mixed with the quantitative statistical information (Fig. 5G) and the outcomes of the ALP exercise assay (Fig. 5H), this proved that the CTAG/GelMA hydrogel performed a major function in contributing to early bone formation. There was calcium deposition on the hydrogels of all teams in line with ARS staining (Fig. 5C, D); nevertheless, the calcium nodules within the CTAG/GelMA group have been extra in depth and extra quite a few than that within the different hydrogel group. Though the distinction between the GelMA and CTAG/GelMA teams within the ALP staining outcomes was not vital, the ARS staining of the GelMA group was far inferior to that of the CTAG/GelMA group, which can be primarily as a result of simple degradation of the pure GelMA hydrogel. The formation of mineralized nodules is a vital operate of osteoblasts, and ARS staining supported the osteogenic properties of the CTAG/GelMA hydrogels.
To additional assess the osteogenic differentiation of BMSCs, immunofluorescence staining for BMP-2 and OCN was carried out. The fluorescence pictures confirmed that the fluorescence depth was larger within the CTAG/GelMA and GelMA teams than within the GO/GelMA group (Fig. 5E, F), which could nonetheless be affected by the poisonous impact of extra GO. The CTAG/GelMA group confirmed probably the most potent expression together with the quantitative statistics (Fig. 5J, Ok), indicating that this group performed probably the most important function in selling osteogenic differentiation. Undoubtedly, the main function is the sluggish launch of Ca2+, which accelerates the osteogenic differentiation of MSCs and regulates the upregulation of BMP-2, OCN, and osteoblastin (OPN) expression [28]. Furthermore, enhancing the extracellular Ca2+ focus can amplify mobile Ca2+ inflow to boost osteogenic differentiation [28]. Due to this fact, CTAG is an appropriate materials for bone restore, and the slow-release Ca2+ from CTAG/GelMA hydrogel performs an essential function in bone reworking [28].
Osteoclasts are multinucleated large cells shaped by the fusion of BMDMs, and osteoclast differentiation markers embrace CTR, TRAP, carbonic anhydrase II (CAII), and cathepsin Ok (cath Ok) [56]. Amongst them, CTR was induced throughout osteoclast formation and was extremely expressed earlier than cell fusion and multinucleated osteoclast formation [57], whereas TRAP was a marker of early osteoclast differentiation [58]. Therefore, the protein expression of CTR and TRAP was analyzed to research the impact of hydrogel on the differentiation of BMDMs to osteoclasts. The outcomes of CTR fluorescence staining confirmed an general lowering development in comparison with the management ( no hydrogel), with the bottom expression within the CTAG/GelMA group, suggesting that it inhibited osteoclast differentiation (Fig. 5M, L). In contrast with the opposite teams, the GelMA group confirmed a extra mature state with a number of TRAP-staining constructive multinucleated cells (Fig. 5N). The TRAP-stained constructive cells within the CTAG/GelMA group have been considerably smaller and fewer, indicating a extra substantial inhibitory impact on osteoclast differentiation than the opposite hydrogel. The emergence of plentiful osteoclasts throughout bone restore will induce extreme bone resorption habits, destabilizing bone formation [59]. Due to this fact, inhibition of osteoclast differentiation by CTAG/GelMA hydrogel is helpful to bone formation stability. At the moment, there are comparatively few research on the results of hydrogels on osteoclasts in vitro, and our method from such a perspective not solely enriches the osteogenic research but additionally illustrates the multifunctional function of hydrogels.
Osteogenic and osteoclastic differentiation of hydrogel. (A, B) ALP staining. (C, D) ARS staining. (E, F) Immunofluorescence staining of BMP-2 and OCN. (G) ALP exercise assay. (H, I) Quantitative evaluation of ALP and ARS staining. (J, Ok) Quantitative evaluation of BMP-2 and OCN immunofluorescence staining. (L, M) Immunofluorescence staining and quantitative evaluation of CTR immunofluorescence staining. (N) TRAP staining of BMDMs-derived osteoclasts. (NS, no vital distinction; *, P < 0.05; **, P < 0.01; ***, P < 0.001)
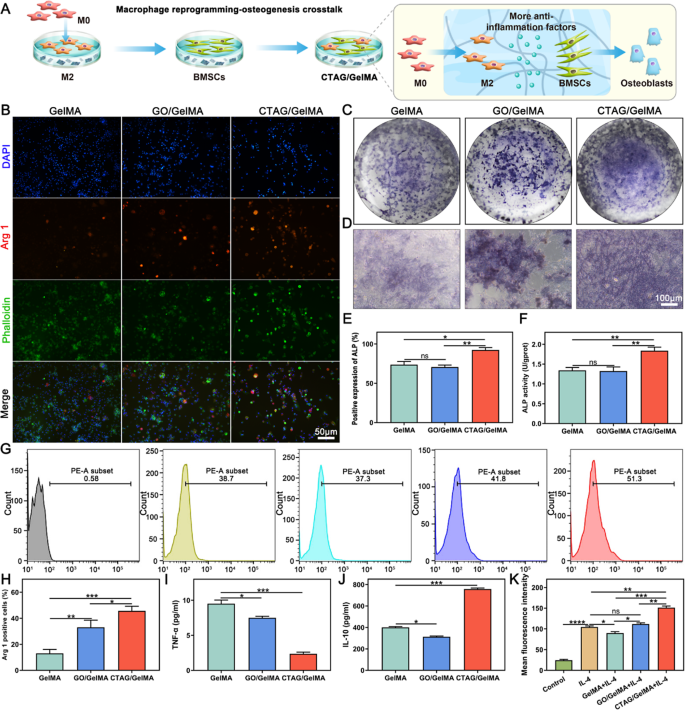
CTAG/GelMA hydrogels orchestrate macrophage reprogramming with pro-osteogenesis in vitro
The method of extraction and identification of BMDMs is described within the Supporting Info (Fig. S8). Macrophages regulate tissue regeneration and reworking within the bone immune microenvironment via polarization (M1 or M2) [60]. Arg 1 and CD206 are broadly used as classical markers for outlining M2 macrophage polarization [61]. We noticed a major expression of mobile Arg 1 within the CTAG/GelMA group, indicating that it accelerated macrophage differentiation in direction of M2 macrophages (Fig. 6B, H). The outcomes of the movement cytometry evaluation (Fig. 6G, Ok) additionally confirmed the same development, with the CD206 expression degree within the CTAG/GelMA group being considerably larger than that of the opposite teams. The typical fluorescence depth of the GelMA group within the movement cytometry statistics was smaller than that of the IL-4 group, suggesting that CTAG performed a vital function in CTAG/GelMA hydrogels and that the addition of CTAG promotes the polarization of M2 macrophages. Thus, CTAG/GelMA hydrogel may coordinate macrophage reprogramming. On this foundation, we co-cultured reprogrammed M2 macrophages with BMSCs to research the macrophage reprogramming-osteogenesis crosstalk (Fig. 6A). Based mostly on the outcomes of ALP staining and ALP exercise assay after 7 days of co-culture, we noticed that the best ALP expression was within the CTAG/GelMA group (Fig. 6C-F). Furthermore, in contrast with the group of BMSCs cultured on hydrogel alone (Fig. 5A-B), ALP expression was enhanced in all corresponding teams of BMSCs co-cultured with M2 macrophages (Fig. 6C-F); due to this fact, this means that macrophage reprogramming promotes osteogenic differentiation of BMSCs. Specifically, the GO/GelMA group confirmed a distinguished enchancment in osteogenesis, most likely as a result of macrophage reprogramming inhibited the poisonous results of GO, thus restoring osteogenesis on this group.
As well as, we examined the secretion of TNF-α and IL-10 throughout macrophage reprogramming-osteogenic differentiation. ELISA outcomes confirmed that TNF-α was considerably decreased and IL-10 was elevated dramatically within the CTAG/GelMA group (Fig. 6I, J). These two inflammatory elements are primarily produced by the M1 and M2 phenotypes, respectively, indicating that macrophage reprogramming enhances osteogenic differentiation of BMSCs by regulating the secretion of inflammatory elements. This reveals modulation of macrophage reprogramming-osteogenic crosstalk, reflecting the significance of bettering the immune microenvironment throughout bone restore. With the event of osteoimmunology, rising numbers of research have confirmed that M2 macrophages promote bone regeneration and vascular restore whereas eradicating broken cells, particles, and useless cells [12]. IL-10, which is extremely expressed in M2 macrophages, has been broadly demonstrated to cut back the synthesis of pro-inflammatory cytokines [33, 62]. IL-10 has additionally been recommended to be an important regulator of bone homeostasis and inflammatory circumstances [13]. M2 macrophages could speed up osteogenesis in an IL-10-dependent method, and IL-10 upregulation promotes osteogenic differentiation of BMSCs [63]. Furthermore, M2 macrophages inhibit the early formation of osteoclasts via proximal and paracrine signaling whereas selling osteogenesis in MSCs [62]. Thus, CTAG/GelMA hydrogels orchestrate macrophage reprogramming to play a central function in bone regeneration.
Macrophage reprogramming-osteogenesis crosstalk results of CTAG/GelMA hydrogels. (A) Schematic illustration of the macrophage reprogramming-osteogenesis crosstalk. (B) Arg 1 immunofluorescence staining. (C, D) ALP staining of BMSCs and macrophages co-cultured on hydrogel floor. (E) ALP exercise assay. (F) Quantification of ALP staining. (G) Stream cytometry outcomes of share of CD206+ cells. (H) Quantification of Arg 1 fluorescent staining. (I, J) ELISA for TNF-α and IL-10. (Ok) Imply fluorescence depth of movement cytometric assays. (NS, no vital distinction; *, P < 0.05; **, P < 0.01; ***, P < 0.001)
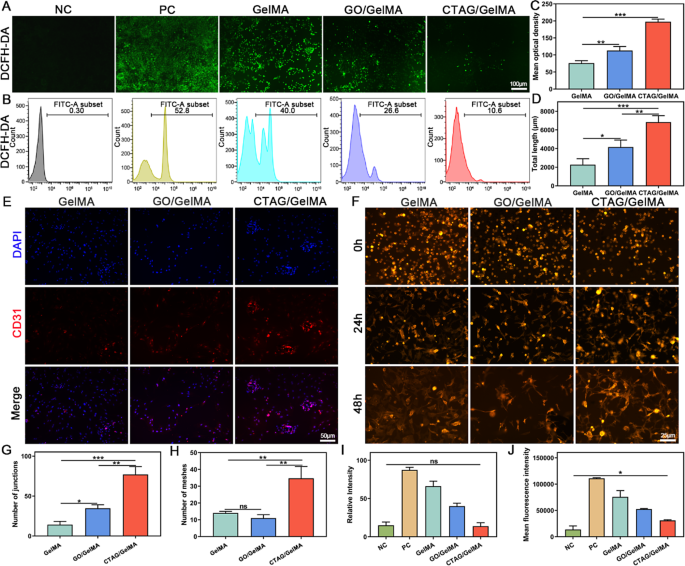
ROS-scavenging and pro-angiogenic capability of the CTAG/GelMA hydrogels in vitro
Research have confirmed that vascular rupture on the website of bone defects results in native hypoxia [64]. Additional, the hypoxic microenvironment within the bone defect area induces intracellular ROS overexpression, affecting bone-associated cell formation and purposeful regulation [65]. Due to this fact, regulating the metabolism of ROS within the bone microenvironment and vascular restore is helpful to osteogenic restore. The in vitro intracellular ROS assay outcomes confirmed that intracellular ROS ranges have been low and undetectable below regular circumstances, whereas intracellular ROS ranges have been considerably elevated after H2O2 stimulation (Fig. 7A, I). Nevertheless, the fluorescence depth of DCFH-DA was diminished in all teams of hydrogels, and the CTAG/GelMA group had probably the most discount. Stream cytometry assays displayed the identical development (Fig. 7B, J), and the proportion of DCFH-DA regularly decreased from 52.8 to 40.0%, 26.6%, and 10.6% after H2O2 stimulation. These outcomes demonstrated that CTAG/GelMA hydrogel retained superior ROS-scavenging properties, attributed to the stable scavenging impact of TA on H2O2 and phenolic free radicals, defending cells from oxidative harm [66]. Excessive ranges of ROS may cause apoptosis, which is dangerous to numerous cells, together with macrophages [67]. Apart from, ROS is without doubt one of the elements regulating the M1/M2 macrophage transition, and reducing native ROS ranges facilitates macrophage transition from the M1 phenotype to the M2 phenotype [68], according to our macrophage reprogramming. Due to this fact, CTAG scavenges oxidative free radicals and protects cells from oxidative harm whereas selling macrophage switching to the M2 phenotype, contributing to the anti-inflammatory and additional bone-repair results of CTAG/GelMA hydrogel.
As well as, the impact of hydrogel on the vascular differentiation of BMSCs was mirrored within the staining of the endothelial cell marker CD31, which was considerably expressed within the CTAG/GelMA group (Fig. 7C, E). In the meantime, the tube formation by HUVECs exhibited {that a} clear tube construction was shaped on the floor of the CTAG/GelMA group relative to the unfinished or sparse tube community of the opposite teams (Fig. 7F). The full size, variety of junctions, and grasp nodes of every tube formation have been additional quantitatively measured utilizing ImageJ software program. The CTAG/GelMA group had the best size, and the numbers of junctions and grasp nodes have been at the least two occasions these of the opposite two teams (Fig. 7D, G, H). The outcomes demonstrated that the CTAG/GelMA hydrogels have sound angiogenic results. It has been beforehand reported that elevated intracellular Ca2+ focus performs a vital function in vascular endothelial cells [69]. Endothelial Ca2+ alerts drive angiogenesis by recruiting a number of Ca2+-sensitive decoders responding to pro-angiogenic alerts, resembling VEGF, fundamental fibroblast development issue (bFGF), and angiopoietins [36, 70]. Due to this fact, CTAG/GelMA hydrogels containing CTAG can promote angiogenesis by stimulating the proliferation of endothelial progenitor cells, tube formation, and neointima formation.
Anti-ROS ranges and angiogenic properties of CTAG/GelMA hydrogels. (A, B) DCFH-DA fluorescence detection and cell movement assay. (C) Quantitative evaluation of CD31 immunofluorescence assay. (D, G, H) Statistical outcomes of complete vessel size, variety of junctions, and variety of grasp nodes. (E) Immunofluorescence of CD31. (F) Consultant pictures of tube formation assays of HUVECs. (I) Quantitative evaluation of DCFH-DA fluorescence assay. (J) Imply fluorescence depth statistics for movement cytometry. (NS, no vital distinction; *, P < 0.05; **, P < 0.01; ***, P < 0.001)
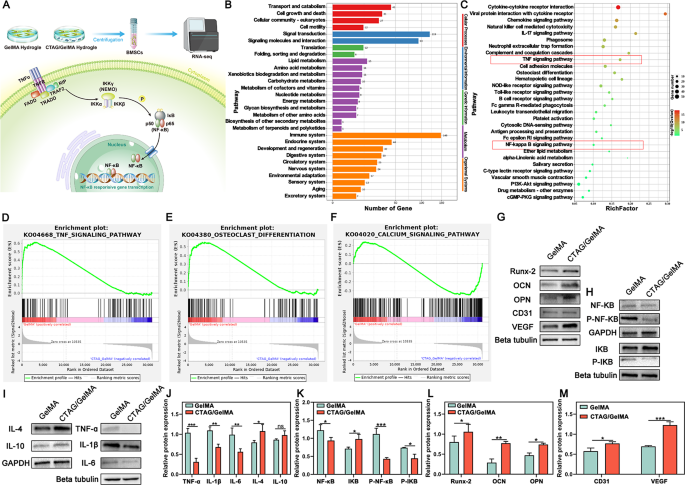
Results of CTAG/GelMA hydrogels on BMSCs analyzed by transcriptomics
To additional discover the regulatory results of CTAG/GelMA hydrogels on BMSCs and their potential mechanisms, we co-cultured cells on the surfaces of hydrogels for 10 days and carried out RNA-seq, as described within the schematic diagram (Fig. 8A). The volcano and warmth maps (Fig. S7A, C) confirmed that there have been 870 differentially expressed genes (DEGs) within the CTAG/GelMA group in comparison with the GelMA group, of which 155 have been elevated and 715 have been decreased (FDR < 0.05, log2FC > 1 or log2FC<-1). Gene ontology evaluation (Fig. S7B and D) confirmed that these DEGs have been primarily related to immune response, irritation regulation, and response to exterior organic stimuli, presumably affected by CTAG/GelMA hydrogel. Due to this fact, we mixed the outcomes of the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment evaluation of DEGs (Fig. 8B) and chosen the highest 30 signaling pathways (Fig. 8C). The outcomes confirmed many signaling pathways have been associated to the immune system, resembling TNF, NF-𝜅B, IL-17, toll-like receptors, and different signaling pathway proteins. Gene set enrichment analyses (GSEAs) additional demonstrated that the CTAG/GelMA hydrogel diminished the enrichment of inflammatory signaling pathways (Fig. 8D), diminished the enrichment of osteoclastic signaling pathways (Fig. 8E), and elevated the enrichment of calcium signaling pathways (Fig. 8F). Amongst them, it has been reported that inhibition of the NF-𝜅B signaling pathway can inhibit the formation of RANKL-induced multinucleated osteoclasts and improve H-vessel formation [71]. In the meantime, the calcium signaling pathway was enriched, suggesting that the osteogenic impact of CTAG/GelMA hydrogel can also be associated to activation of the calcium signaling pathway. Calcium signaling can keep stem cell pluripotency by regulating transitions between cell cycles, and this signaling is important for cell proliferation, differentiation, apoptosis, and plenty of different processes [72]. The outcomes point out that CTAG introduction is significant for the regulation of the osteogenic microenvironment in bone defects, revealing the intrinsic regulatory mechanisms of CTAG/GelMA hydrogels on immunomodulation, osteoclast differentiation, and osteoblast differentiation.
Nuclear factor-kB (NF-kB) is a key intracellular nuclear transcription consider organismal immune response and regulates apoptosis and stress response [73]. The TNF household (resembling TNF-α, and TNF-β) primarily regulates the classical NF-κB signaling pathway [74]. Research have demonstrated that TNF-α overexpression can instantly activate the NF-κB pathway, additional stimulating the manufacturing of pro-inflammatory cytokines and inflicting inflammatory responses [75]. On the similar time, TNF-α can stimulate the secretion of IL-6 and different inflammatory elements from BMDMs by activating the NF-κB transcription issue signaling pathway and protease pathway, which induce or worsen the inflammatory course of [76]. To confirm that CTAG/GelMA hydrogel could inhibit the inflammatory response primarily via the NF-𝜅B signaling pathway, we detected NF-𝜅B-related proteins by western blot evaluation. NF-𝜅B expression was diminished within the CTAG/GelMA group, and the NF-𝜅B and IKB phosphorylation have been inhibited considerably (Fig. 8H, Ok). Subsequently, CTAG/GelMA hydrogel downregulated TNF-ɑ, IL-1β, and IL-6 inflammation-associated cytokine expression and upregulated IL-4 and IL-10 expression (Fig. 8I, J). IL-1β and TNF-α are among the many inflammatory cytokines secreted by M1 macrophages. These cytokines hinder osteoblasts from synthesizing alkaline phosphate and impair extracellular bone matrix secretion and mineralization [77]. In distinction, IL-4 is an inducer of M2 macrophages and accelerates bone cell proliferation [78]. From such a perspective, macrophage reprogramming regulation throughout bone formation is crucial for osteogenesis. Mixed with the outcomes of Western blot evaluation on osteogenesis and angiogenesis-related genes (Fig. 8G, and 8L, M), CTAG/GelMA hydrogel promotes osteogenesis and angiogenesis-related gene expression in BMSCs. In conclusion, CTAG/GelMA hydrogel may improve osteogenesis regulation by regulating inflammatory elements.
CTAG/GelMA hydrogels modulate anti-inflammatory-related gene expression and osteogenic or angiogenic-related protein ranges in vitro. (A) Schematic illustration of the RNA-seq assay. (B, C) Differential gene KEGG enrichment evaluation categorized histograms in addition to enrichment bubble plots. (D, E, F) GSEAs of associated signaling pathways. (G, H, I) Expression ranges of related protein bands. (J, Ok, L, M) Quantitative evaluation of the expression ranges for related proteins. (NS, no vital distinction; *, P < 0.05; **, P < 0.01; ***, P < 0.001)
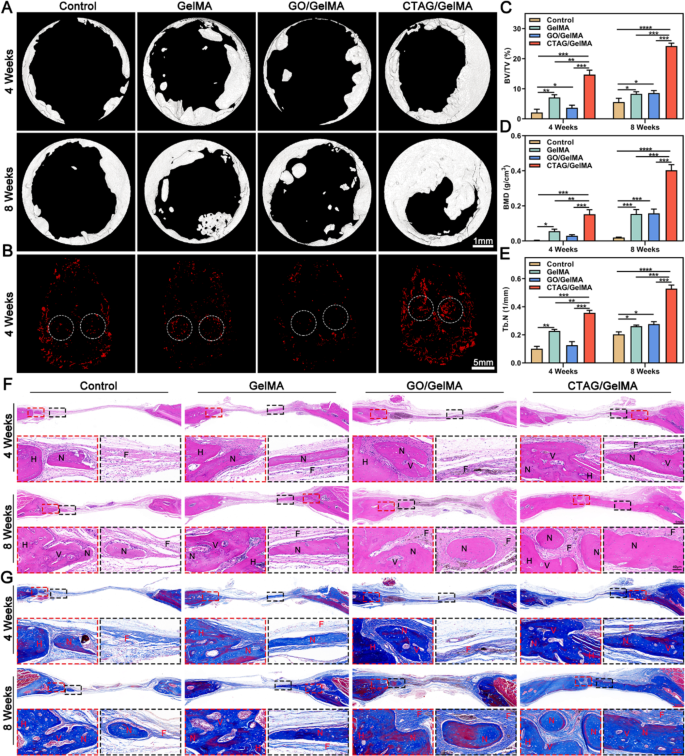
Bone reconstruction and immunomodulation of CTAG/GelMA hydrogel in vivo
A critical-sized cranium defect mannequin (Φ = 5 mm) in rats was chosen to guage additional the regulatory impact of the CTAG/GelMA hydrogel on bone regeneration (Fig. S10). The consultant pictures by Micro-CT (Fig. 9A) recommended that the newly shaped bone tissue may barely be noticed within the management group at 4 weeks, whereas some newly shaped bone was seen across the margins of the defect within the CTAG/GelMA group. At 8 weeks, the osteogenic tendency was extra pronounced within the CTAG/GelMA group in comparison with the opposite teams, with probably the most appreciable improve within the space coated with new bone. Quantitative evaluation of Micro-CT additional confirmed that the CTAG/GelMA group had larger BV/TV values (14.715 ± 0.844%, 4 w; 24.188 ± 1.554%, 8 w), BMD (0.152 ± 0.015 g/cm3, 4 w; 0.403 ± 0.019 g/cm3, 8 w) and Tb. N (0.357 ± 0.011 1/mm, 4 w; 0.529 ± 0.015 1/mm, 8 w), demonstrating that CTAG/GelMA hydrogel promoted rat cranial regeneration (Fig. 9C-E). Based mostly on 3D reconstruction of neovascularization on the location of the cranial defect at 4 weeks postoperatively, the CTAG/GelMA group had the best variety of neovessels across the bone defect (Fig. 9B). It indicated that the pro-vessel formation skill of CTAG/GelMA hydrogel was significantly vital, which is according to the outcomes of angiogenesis experiments in vitro (Fig. 7E, F).
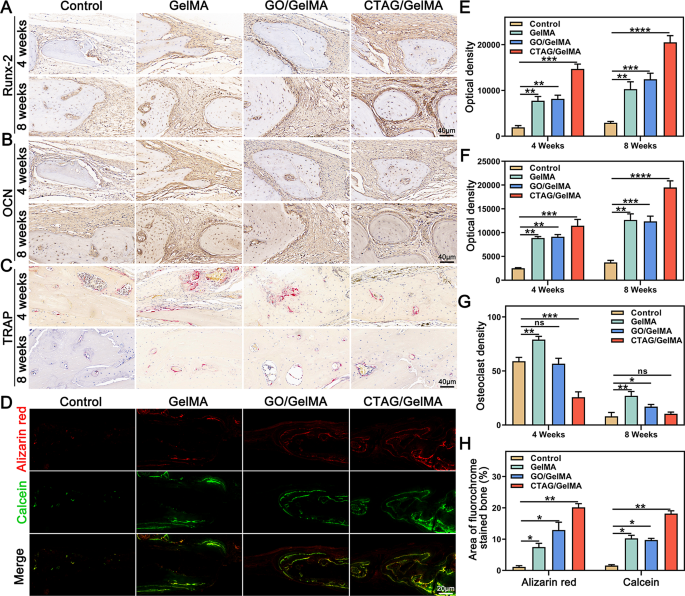
After Micro-CT scanning, HE and Masson staining have been used to watch the formation of latest bone on the cranial defect website (Fig. 9F, G). Within the management group, the defect space was dominated by fibrous tissue formation bridging the sting of the host bone at 4 and eight weeks, indicating poor bone regeneration potential. In distinction, regenerated lamellar bone was seen within the GelMA and GO/GelMA teams, accompanied by new bone cavities and blood vessels. Furthermore, the CTAG/GelMA group had one of the best therapeutic outcomes, with the defect space virtually totally occupied by dense and mature neoblastic bone tissue, and the thickness was just like that of the unique bone at eight weeks postoperatively. We then used immunohistochemical staining to evaluate the composite hydrogel osteogenic results additional. In contrast with the management group, Runx-2, and OCN-positive areas have been probably the most intense within the CTAG/GelMA group (Fig. 10A, B, E and F). In the meantime, the results of various hydrogels on new bone formation and mineralization charge in vivo have been noticed by multi-color sequential fluorescent labeling (Fig. 10D, H). The expression of ARS staining within the CTAG/GelMA group was larger than within the different teams, indicating that new bone formation was most distinguished throughout the early levels of bone regeneration. The expression of calcein fluorescence within the GelMA and GO/GelMA teams was barely larger than within the management group. In distinction, calcein fluorescence was the best within the CTAG/GelMA group, confirming that the CTAG/GelMA group had the best mineralization charge. Apart from, TRAP staining confirmed virtually no mature osteoclasts within the CTAG/GelMA group. Specifically, TRAP-positive osteoclasts have been diminished by 50% in contrast with within the GelMA group (Fig. 10C, G). In conclusion, CTAG/GelMA hydrogels are superior in selling osteogenesis in vivo and lowering bone loss by inhibiting osteoclast formation.
CTAG/GelMA hydrogel enhances in situ regeneration of cranial bone in a rat cranial defect mannequin. (A) Typical pictures of cranial Micro-CT in rats with bone defects. (B) Consultant pictures of three-dimensional reconstruction of vessels. (C, D, E) BV/TV, BMD, and Tb. N outcomes have been calculated based mostly on Micro-CT. (F, G) HE staining and Masson staining of postoperative regenerated bone. N as new bone, H indicated as host bone, V because the vessel, and F as fibrous tissue (NS, no vital distinction; *, P < 0.05; **, P < 0.01; ***, P < 0.001)
In vivo osteogenic results of CTAG/GelMA hydrogels. (A, B) Consultant immunohistochemical pictures of Runx2 and OCN. (C) Consultant pictures of TRAP staining. (D) Alizarin Purple and calcein consultant pictures. (E, F) Immunohistochemical optical density of Runx-2 and OCN. (G) Quantitative evaluation of TRAP-stained osteoclast density. (H) Quantifying the world of latest bone formation labeled by alizarin pink and calcein. (NS, no vital distinction; *, P < 0.05; **, P < 0.01; ***, P < 0.001)
Immunomodulation, biosafety, and biodegradability of CTAG/GelMA hydrogel in vivo
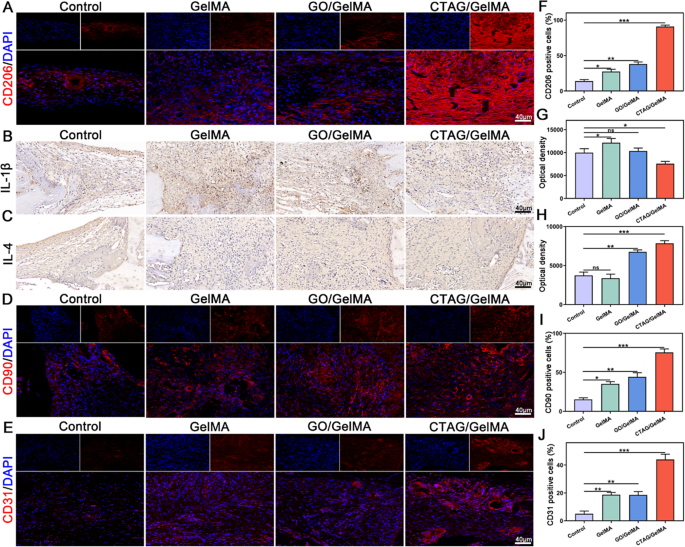
It’s more and more demonstrated that energetic modulation of the immune system promotes tissue restore, significantly macrophages, that are able to secreting a variety of cytokines that instantly affect the inflammatory response and MSCs recruitment and differentiation [4, 8, 79]. Persistent irritation triggers apoptosis of MSCs over time. Thus, the important thing to rising bone regeneration is to transform macrophages to the M2 phenotype on the acceptable time level [12, 80]. Anti-inflammatory cytokines from M2 cells can even stabilize angiogenesis and coordinate ECM meeting [81]. To analyze the function of CTAG/GelMA hydrogel in orchestrating macrophage reprogramming-osteogenesis crosstalk in vivo, CD206 fluorescent staining was used to detect macrophage phenotypes in cranial defects seven days after surgical procedure. The expression of CD206 was larger within the CTAG/GelMA group than within the different teams, representing the upper variety of M2 macrophages within the damage space (Fig. 11A, F). The outcomes of immunohistochemical staining for inflammatory elements (Fig. 11B, C and G, H) additional confirmed that the CTAG/GelMA hydrogel had a very good anti-inflammatory modulating impact, selling the elevation of the early anti-inflammatory issue IL-4 and the discount of the pro-inflammatory issue IL-1β. It means that CTAG/GelMA hydrogel promotes macrophage polarization in direction of M2 macrophages, contributing to regulation of the bone immune atmosphere. Then, CD90 (MSC floor marker) fluorescence staining was carried out in line with earlier experiments [40]. The CTAG/GelMA group had the best CD90-positive expression, indicating that early MSCs may be recruited in massive numbers to provoke bone restore at an early stage (Fig. 11D, I). Moreover, the outcomes of host vascular endothelial cell (CD31+) staining at 4 weeks postoperatively reconfirmed the pro-angiogenic impact of CTAG/GelMA hydrogel (Fig. 11E, J). Mixed with the report that M2 macrophages can secrete VEGF to additional promote angiogenesis [12], the CTAG/GelMA hydrogel is extremely efficient in selling angiogenesis. In abstract, CTAG/GelMA hydrogel promotes macrophage reprogramming to the M2 phenotype early in vivo, accelerates MSCs recruitment, and promotes bone and vascular restore.
To confirm the biosafety and degradability of the composite hydrogels in animals, GelMA, GO/GelMA, and CTAG/GelMA hydrogels have been implanted subcutaneously on the backs of rats and samples have been taken for statement at 1, 4, and eight weeks after implantation. The degradation pictures and curves (Fig. S9A, D) at completely different time factors demonstrated that the CTAG/GelMA hydrogel degraded on the slowest charge, suggesting that 4 mg/mL CTAG addition extended the motion time of the hydrogel, thereby favoring the therapeutic of the bone. That is according to the beforehand characterised outcomes, the place enhanced mechanical energy is related to a corresponding improve in degradation time. As well as, HE staining and CD68 fluorescence staining outcomes (Fig. S9B, C, and S9E) confirmed that each one teams, excluding the CTAG/GelMA group, had a comparatively excessive inflammatory response at one week of implantation, with slight disruption of the association of myofilaments and collagen. Nonetheless, the pores and skin tissue was virtually regular at eight weeks. The observations recommended that subcutaneous tissues of all teams may return to regular because the hydrogel degraded, which additionally confirmed the immunomodulatory impact of CTAG/GelMA hydrogel. Furthermore, the HE staining outcomes of the foremost organs (Fig. S11) revealed no vital distinction between teams, additional validating the biosafety of the composite hydrogel. General, CTAG/GelMA hydrogel has good degradability and biosafety in vivo. The CTAG/GelMA hydrogel exerted mixed pro-immunomodulatory, osteogenic, and angiogenic results in vivo.
In vivo anti-inflammatory and angiogenesis-related results of CTAG/GelMA hydrogels. (A) Consultant pictures of CD206 immunofluorescence staining. (B, C) Consultant immunohistochemical photos of IL-1β and IL-4. (D, E) Immunofluorescence staining of CD90 and CD31. (F) Quantification of CD206 immunofluorescence staining. (G, H) Immunohistochemical optical density of IL-1β and IL-4. (I, J) Quantitative evaluation of CD90 and CD31 immunofluorescence staining. (NS, no vital distinction; *, P < 0.05; **, P < 0.01; ***, P < 0.001)