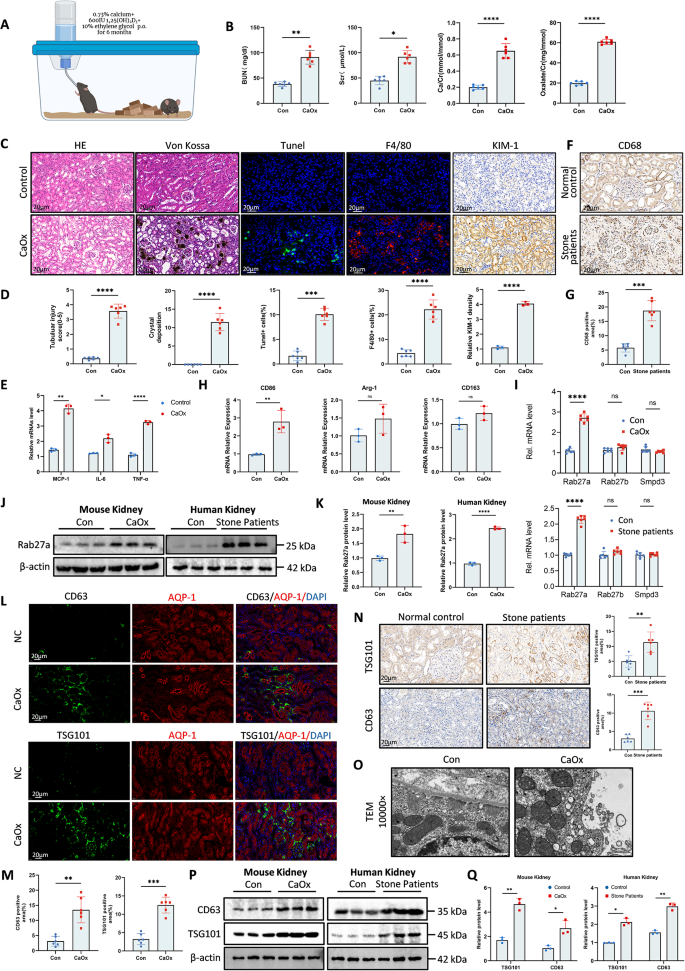
Macrophage infiltration was elevated in kidney tissue of CaOx crystal deposition mice and stone sufferers
We established a mouse mannequin utilizing a vitamin D-supplemented high-calcium weight-reduction plan (Fig. 1A) in response to beforehand reported strategies [25]. Blood urea nitrogen (BUN) and serum creatinine (Scr) ranges, and urinary calcium and oxalate ranges elevated had been elevated remarkably on this mannequin (Fig. 1B). HE and Von Kossa staining indicated an aggravated renal cortical harm and elevated calcium crystal deposition in renal tubular lumens (Fig. 1C-D), suggesting the profitable institution of the mouse mannequin of nephrolithiasis. Tunel and F4/80 staining demonstrated the improved macrophage infiltration within the kidneys of nephrolithiasis mice (Fig. 1C-D). MCP-1, IL-6 and TNF-α had been considerably up-regulated in kidney stone mice (Fig. 1E). The immunohistochemical constructive stage of CD68 within the kidney tissue of nephrolithiasis sufferers is considerably elevated (Fig. 1F-G). Within the kidneys of mice mannequin, mRNA ranges of macrophage markers recommended that pro-inflammatory M1 macrophages had been the predominant elevated (Fig. 1H). These outcomes recommend that CaOx crystal deposition induces vital macrophage infiltration, with a predominance of pro-inflammatory M1 macrophages.
Macrophage infiltration and exosomes secretion was elevated in kidney tissue of CaOx crystal deposition mice and stone sufferers. A Schematic diagram of kidney stone mouse mannequin. B BUN, SCr, Ca and oxalate ranges in mice. C–D Consultant photos of HE, Von Kossa, Tunel staining, F4/80 and KIM-1 (scale bar = 20 μm) and quantitative evaluation. E mRNA ranges of inflammatory chemokines MCP-1, IL-6, TNF-α in management and stone mouse kidneys. F–G Consultant IHC photos of CD68 in regular management and stone sufferers kidneys and quantitative evaluation. Scale bar = 20 μm. H mRNA ranges of macrophage markers CD86, ARG-1 and CD163. I Expression ranges of genes associated to exosome secretion of mice and human. J–Okay Protein ranges of Rab27a in mouse kidney and human kidney. L–M Consultant IF photos and quantitative outcomes of CD63 and TSG101 and proximal RTECs marker AQP-1 in mouse kidney tissues. N Consultant IHC photos and quantitative outcomes of CD63 and TSG101. O Consultant TEM photos displaying extracellular vesicles secreted by RTECs at 12d after modelling. Scale bars = 500 nm. P–Q Consultant western blot photos and quantitative evaluation confirmed the expression ranges of CD63 and TSG101 within the management and CaOx mice, and management and stone sufferers kidneys. Knowledge are proven as imply ± SEM. P-values had been calculated from two-tailed impartial t-tests.*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Secretion of extracellular vesicles from kidney proximal tubules was elevated in kidney stones mice and sufferers
To analyze the position of extracellular vesicle (EV) secretion in nephrolithiasis, we examined 3 genes, Rab27a, Rab27b and Smpd3 (Fig. 1I), that are associated to extracellular vesicle secretion in mice and human renal tissues [26, 27]. Amongst these, solely Rab27a was considerably up-regulated (Fig. 1J-Okay). Immunofluorescence confirmed that CD63- and TSG101- labelled EVs had been primarily localized round proximal renal tubular epithelial cells (Fig. 1L-M). In kidney tissues from nephrolithiasis sufferers, immunohistochemical (IHC) evaluation of CD63 and TSG101 revealed a major improve in EV secretion in comparison with controls (Fig. 1N). Transmission electron microscopy (TEM) additional confirmed the next variety of EV our bodies within the proximal renal tubular epithelial cells of mouse kidneys (Fig. 1O). Western blot outcomes of CD63 and TSG101 in each mouse and human kidney tissues supported the above conclusions (Fig. 1P-Q). These outcomes illustrated that the secretion of extracellular vesicles was elevated within the technique of nephrolithiasis, suggesting a possible position for EVs within the pathophysiology of kidney stone illness.
CaOx-stimulated exosomes from TCMK-1 cells promoted M1 polarization
TCMK-1 cells had been stimulated by 150mmol/ml CaOx, with or with out the exosome secretion inhibitor GW4869, for twenty-four h. The medium was then changed with exosome-free medium for a further 24 h. The exosomes within the conditioned medium had been collected to stimulate RAW264.7 cells (Fig. 2A). Characterization of exosome markers (CD63, TSG101, CD81 and adverse marker Calnexin) by western blot, morphology and dimension by transmission electron microscopy (TEM) and nanoparticle monitoring evaluation (NTA) confirmed the profitable isolation of control-exosomes (Ctrl-exo) and CaOx-exosomes (CaOx-exo) (Fig. 2B-C). As well as, the focus of CaOx-exo was considerably increased than Ctrl-exo (Fig. 2D-E), which can be attributed to the elevated expression of Rab27a. PKH26-labelled exosomes had been co-cultured with RAW264.7 cells, confirming that RAW264.7 cells might internalize these exosomes (Fig. 2F). CaOx considerably enhanced exosome secretion from TCMK-1 cells, and macrophages handled with CaOx-exo exhibited a shift towards the pro-inflammatory M1 phenotype. In distinction, macrophages handled with exosomes derived from TCMK-1 cells uncovered to each GW4869 and CaOx confirmed decreased M1 polarization (Fig. 2G-I, Fig S1A).
CaOx-stimulated exosomes from TCMK-1 promoted M1 polarization. A Schematic illustration of macrophages handled with exosomes derived from TCMK-1 cells. B Exosomes had been recognized by floor markers. C TEM and NTA photos of exosomes from TCMK-1 cells. Scale bars = 100 nm. D Exosome focus was assessed by western blot evaluation of the identical quantity of Ctrl-exo and CaOx-exo. E Density detection of Ctrl-exo and CaOx-exo. F Consultant IF picture of TCMK-1 cell-derived exosomes taken up by RAW264.7 cells (inexperienced for phalloidin and purple for PKH- labelled exosomes). Scale bars = 20 μm. G Consultant western blot of CD63 and TSG101 in TCMK-1 handled with GW4869 and CaOx. H Consultant western blot of iNOS and ARG-1 in RAW264.7 handled with exosomes from G teams. I Consultant IF photos of M1 macrophage markers F4/80 and iNOS in 4 teams of macrophages. J Consultant IF photos displaying the degrees of TSG101 and iNOS within the kidneys of stone-induced management and Rab27a-/- mice. Okay Consultant circulate cytometry outcomes confirmed 4 teams of iNOS + F4/80 + and ARG-1 + F4/80 + macrophages, which signify modifications in M1 and M2 ranges. L Relative mRNA ranges of consultant markers of M1 and M2 macrophages, M1 markers had been considerably modified by CaOx stimulation and GW4869. M Cytokines produced by Ctrl-exo and CaOx-exo-treated macrophages had been assayed by ELISA. Knowledge are proven as imply ± SEM. In D, E, M, p-values had been calculated from two-tailed impartial t-tests. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
To additional make clear the connection between exosomes and kidney irritation in vivo, we constructed Rab27a knockout mice (Rab27a-/-) (Fig. S1B-C). As anticipated, the kidney of Rab27a-/- mice exhibited much less M1 polarization in comparison with than wild-type mice (Fig. 2J, Fig S1D). Movement cytometry additional demonstrated that CaOx-exo promoted a pro-inflammatory M1 phenotype, whereas GW4869 mitigated this impact (Fig. 2Okay). In CaOx-exo-treated macrophages, mRNA ranges of M1 markers (TNF-α, iNOS, CD86) had been considerably upregulated, whereas M2 markers (CD163, IL-10, TGF-β1, Arg-1) had been downregulated (Fig. 2L). RAW264.7 uncovered to CaOx-exo secreted increased ranges of proinflammatory cytokines, equivalent to IL-6, IL-8 and TNF-α, and decrease ranges of anti-inflammatory IL-10, in comparison with Ctrl-exo-treated cells (Fig. 2M). The above findings recommended that CaOx-exo derived from TCMK-1 induced M1 polarization, contributing to a pro-inflammatory surroundings.
Exosomal mir-93-3p concentrating on macrophage NFAT5 Could also be a possible mechanism of kidney harm induced by stones
MiRNAs sequencing of Ctrl-exo and CaOx-exo revealed 57 considerably up-regulated and 46 considerably down-regulated miRNAs (|log2FC|>2.5, P < 0.01) (Fig. 3A). After filtering out low-expression miRNAs and screening for homologous ones, we narrowed the candidates to fifteen miRNAs (Fig. 3B). By intersecting the above 15 candidates with the differentially expressed miRNAs of folic acid-induced kidney harm and fibrosis (GSE61382), and unilateral ureteral obstruction (UUO) (GSE118340), we recognized two potential targets: mmu-miR-27-5p and mmu-miR-93-3p (Fig. 3C). RNA sequencing of macrophages handled with exosomes revealed enrichment of inflammatory pathways and pro-inflammatory issue manufacturing primarily based on KEGG and GO analyses (Fig. 3D, Fig S2A). To pinpoint the important thing pro-inflammatory miRNA, TCMK-1 cells had been transfected with miR-27-5p or miR-93-3p mimics or inhibitors, and the polarization of macrophages was assessed. Transfection of mimics of miR-93-3p however not miR-27a-5p resulted in a major improve of iNOS expression, indicating that the previous was the first contributor to M1 polarization (Fig. 3E-F, Fig S2B-C). After filtering, we recognized NFAT5 because the potential goal gene of miR-93-3p utilizing 5 miRNA goal gene prediction instruments (TargetScan, miRDB, miRWalk, miRmap and Tarbase) (Fig. 3G). Nuclear issue of activated T-cells 5 (NFAT5) is understood to play a key position in irritation and immune responses triggered by damage-associated molecular patterns (DAMPs). Notably, earlier research have proven that NFAT5 contributes to the development of power arthritis by selling macrophage polarization [28]. RT-qPCR evaluation confirmed that NFAT5 expression was decreased in macrophages handled with CaOx-exo (Fig. 3H). Bioinformatics evaluation revealed a complementary binding sequence between miR-93-3p and the three′ untranslated area (3′UTR) of NFAT5 (Fig. 3I). Cells co-transfected with miR-93-3p mimics and a luciferase reporter plasmid containing WT NAFT5 3’UTR confirmed decrease luciferase exercise (Fig. 3J). IHC and Western blot analyses confirmed that NFAT5 expression was considerably decrease within the kidney tissues of CaOx mice and nephrolithiasis sufferers than in controls (Fig. 3Okay-L, Fig S2D). Based mostly on these findings, we chosen miR-93-3p for additional analysis and concluded that CaOx-exo regulates renal harm brought on by kidney stones by exosomal-miR-93-3p/NFAT5 axis.
Exosomal mir-93-3p concentrating on macrophage NFAT5 could also be a possible mechanism of kidney harm induced by stones. A Volcano plot of miRNA sequencing. B Warmth map of miRNA sequencing. C 2 candidate miRNAs had been obtained after intersection of genes within the warmth map with GSE61328 and GSE118340. D KEGG enrichment analyses had been carried out on the RNA sequencing outcomes of CaOx-exo and Ctrl-exo-treated macrophages. E The mimics and inhibitors of miR-27a-5p and miR-93-3p had been used to confirm their results on macrophage polarization, respectively. G Venn diagram displaying the intersection of potential goal genes of miR-93-3p predicted by 5 databases: TargetScan, miRDB, Tarbase, miRWalkand miRmap. H Relative mRNA ranges of the 5 genes within the intersection. I The binding website of wild-type NAFT5 with miR-93-3p, and mutant sequence of NFAT5 3ʹ-UTR. J Luciferase exercise of cells cotransfected with mimics-NC or miR-93-3p mimics and NFAT5-WT or NFAT5-Mut luciferase reporter plasmid. Okay Consultant IHC photos indicating NFAT5 expression ranges in mouse kidneys. Scale bars = 20 μm. L The expression ranges of NFAT5 and iNOS in mouse and human kidney tissues had been detected by Western blot. Knowledge are proven as imply ± SEM. In H, P-values had been calculated from two-tailed impartial t-tests. In J, One-way ANOVA with Tukey’s a number of comparisons check. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
The NIK/NF-κB2 pathway promotes M1 polarization and METs formation
The NIK/NF-κB2 pathway within the RNA sequencing outcomes of macrophages emerged as a key space of curiosity (Fig. 3D). Evaluation from the general public database (humphreyslab.com/SingleCell/) revealed that considerably elevated expression NIK(Map3k14) within the kidneys of mice 14 days after UUO surgical procedure. NIK is principally expressed in macrophages and repairing renal tubular epithelial cells (Fig S3) [29]. To additional confirm the regulatory impact of miR-93-3p on NFAT5 and NIK/NF-κB2 pathway, Western blot was carried out to evaluate the degrees of the important thing molecules in RAW264.7 cells. Not surprisingly, NIK and its downstream p52 had been considerably elevated, whereas p100, the precursor of p52, and NFAT5 had been decreased when cells had been stimulated with CaOx-exo and transfected with miR-93-3p mimics. Conversely, when cells had been handled with CaOx-exo and transfected with miR-93-3p inhibitors, NIK and p52 ranges elevated considerably. Co-transfection with LV-NFAT5 additional amplified these modifications (Fig. 4A-B). Moreover, therapy with miR-93-3p inhibitor and/or si-NIK decreased p100 ranges whereas elevated p52 ranges in comparison with the management group (Fig. 4C-D). These findings recommend that miR-93-3p and NIK each act as key regulators in suppressing the NF-κB2 pathway.
The NIK/NF-κB2 pathway promotes M1 polarization and METs formation. A–B Consultant western blot outcomes confirmed the degrees of NFAT5, NIK, p100, p52 and RelB in RAW264.7 cells stimulated with completely different teams of TCMK-1-derived exosomes. C–D Western blot evaluation of NIK, iNOS, p100 and p52 when NIK is knocked down. E–F SYTXO-green staining confirmed the formation of METs, and citH3 + MMP12 + RAW264.7 represented cells present process METosis. Knowledge are proven as imply ± SEM. Two-way ANOVA with Tukey’s a number of comparisons check.*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Along with regulating inflammatory components and pro-inflammatory pathways, we discovered the time period “extracellular house” within the outcomes of KEGG enrichment evaluation (Fig. 3D). Latest research reported a novel type of macrophage loss of life, often called METosis, characterised by the discharge of DNA and proteins into the extracellular house to kind buildings referred to as METs. To verify the presence of METs, we stained extracellular DNA meshwork buildings together with MET markers (citH3 and MMP12). In keeping with the earlier outcomes, IF outcomes confirmed that CaOx-exo-stimulated macrophages had been polarized into M1 phenotype, and METs formation was considerably elevated, which could possibly be reversed by utilizing miR-93-3p inhibitor and si-NIK (Fig. 4E-F). In abstract, CaOx-exo exacerbated irritation and kidney harm induced by kidney stones by selling M1 polarization and METs launch.
NFAT5 repressed NIK/NF-κB2 pathway by way of ubiquitination and degradation of NIK by Akt1/cIAP1/2
We used the STRING database to determine the mediator molecule between NFAT5 and NIK/NF-κB2. 10 molecules had been associated with NFAT5, and amongst them, solely Akt1 was a part of the of NF-κB2 signalling pathway cluster (Fig. 5A). Subsequently, we hypothesized that NFAT5 regulates the NIK/NF-κB2 pathway by Akt1. Western blot evaluation revealed that flattening NFAT5 decreased the degrees of p-Akt1/Akt1 and cIAP1/2 whereas growing NIK ranges (Fig. 5B). si-NFAT5 potentiated the CaOx-exo-induced improve in NIK expression (Fig. 5C). In RAW264.7 cells, Akt1 phosphorylation was inversely correlated with NIK protein ranges in a dose-dependent method (Fig S4A). Given Cycloheximide (CHX) to cells to inhibit protein synthesis, therapy with the Akt1 activator SC79 promotes NIK degradation, whereas NIK exhibited an prolonged half-life within the absence of SC79 (Fig S4B). Ubiquitination is a standard post-translational modification (PTM) concerned in protein degradation, and former research have recognized cIAP1/2 as the first E3 ubiquitin ligases concentrating on NIK for degradation [30, 31]. The general public PTM database additional confirmed the presence of a number of ubiquitination and phosphorylation websites on NIK (Fig S4C). To look at the roles of NFAT5 and Akt1 have an effect on NIK ubiquitination and degradation, cells had been handled with NFAT5 or cIAP1/2 overexpression plasmids, SC79, or proteasome inhibitor MG132, respectively. Overexpression of NFAT5 or cIAP1/2, or therapy with SC79 decreased NIK protein ranges, whereas MG132 co-treatment inhibited NIK degradation (Fig. 5D). We additional examined the degrees of ubiquitination in RAW264.7 in numerous circumstances. Co-immunoprecipitation experiments confirmed that NFAT5 promoted NIK ubiquitination (Fig. 5E). Activation of Akt1 by SC79, or overexpression of cIAP1/2 additionally promoted NIK ubiquitination (Fig. 5F-G). These outcomes reveal that NIK degradation mediated by p-Akt1 is related to NIK ubiquitination. NFAT5 promotes Akt1 phosphorylation, which prompts cIAP1/2. These E3 ubiquitin ligases instantly ubiquitinate NIK, thereby suppressing the NF-κB2 pathway.
NFAT5 repressed NIK/NF-κB2 pathway by way of ubiquitination and degradation of NIK by Akt1/cIAP1/2. A Protein-protein interplay networks for NFAT5 and NIK/ NF-κB2 pathway had been constructed utilizing the STRING on-line database. B Protein ranges of NFAT5, Akt1,p-Akt1, cIAP1/2 and NIK in macrophages transfected with NC or si-NAFT5 had been decided utilizing Western blot. C Consultant photos and quantitative evaluation of IF staining of NFAT5 in RAW264.7 cells in 4 completely different therapy teams. Scale bar = 20 μm. D Protein ranges of NFAT5, Akt1,p-Akt1, cIAP1/2 and NIK in macrophages transfected with Flag-NFAT5, Myc-cIAP1/2 plasmids or utilized with SC79, DMSO or MG132, had been decided utilizing Western blot. E–G TCMK-1 cells had been transfected with completely different plasmids and handled with MG132. Cell lysates had been immunoprecipitated with Myc antibody. Ubiquitinated and complete NIK was detected by immunoblot evaluation of anti-HA and anti-FLAG antibodies. Immunoblot evaluation of NFAT5, Myc (NIK), HA (Ub), and β-actin in whole-cell lysates. Knowledge are proven as imply ± SEM. Two-way ANOVA with Tukey’s a number of comparisons check.*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Contemplating that Akt1 is a canonical kinase and NIK has some phosphorylation modification websites, we carried out CO-IP and located no direct interplay between NIK and p-Akt1 (Fig S4D). In RAW264.7 cells overexpressing NIK or handled with SC79, p-Akt1 elevated p52 protein stage, with out altering NIK phosphorylation (Fig S4E). This can be linked to IKKα activation, one other vital kinase within the NF-κB2 pathway, mediated by Akt1.
Total, our outcomes point out that p-Akt1 promotes cIAP1/2 expression, which in flip ubiquitinates and degrades NIK. NFAT5, performing as an upstream regulator of Akt1, permits NIK to flee from ubiquitination-mediated degradation when down-regulated by exosomal-miR-93-3p, thereby inhibiting the NF-κB2 pathway.
Tubular epithelial cells derived miR-93-3p promotes M1 polarization and METs formation in vivo
To analyze the correlation between proximal tubular epithelia cells derived exosomes and CaOx-induced renal irritation, we carried out an animal research in a CaOx mice mannequin by tail intravenous injection of CaOx-exo or Ctrl-exo (Fig. 6A). CaOx-exo injection considerably exacerbated renal dysfunction in comparison with Ctrl-exo-treated regular management (NC) and CaOx mice (Fig. 6B). TCMK-1-derived exosomes had been labelled with DiR, and their biodistribution was tracked by fluorescence imaging of the lungs, liver, and kidneys at 6, 12, 24, and 48 h post-injection. Fluorescence depth elevated over time, with the very best accumulation within the lungs, adopted by the liver, and relatively decrease ranges within the kidneys in any respect time factors (Fig S5A). After accounting for fluorescence interference from different organs, kidney uptake of exosomes was noticed to extend over time, peaking at 48 h (Fig S5B). Based mostly on these findings, subsequent in vivo experiments centered on the fluorescence depth on the 48-hour time level.
Tubular epithelial cells derived miR-93-3p promotes M1 polarization and METs formation in vivo. A Schematic diagram of the kidney stone mouse mannequin by tail intravenous injection exosomes. B Renal perform in every group of mice. C DIR-labelled exosomes had been noticed in vivo to judge exosome uptake effectivity by the kidneys after modelling. D–E Protein ranges of iNOS, NGAL, KIM-1, citH3, NIK, p100 and p52 in kidneys of various teams. F–G Consultant pathological staining photos, IHC photos and IF photos displaying the expression of kidney harm, crystal deposition, NFAT5, NIK, p-AKT and citH3 + F4/80 + areas. Scale bar = 20 μm. Knowledge are proven as imply ± SEM. Two-way ANOVA with Tukey’s a number of comparisons check.*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Apparently, CaOx-exo injection in CaOx mice resulted in considerably increased exosome uptake within the kidneys in comparison with Ctrl-exo-treated NC mice (Fig. 6C). This implies that CaOx-exo exacerbates pre-existing renal harm brought on by kidney stones, probably by enhanced macrophage phagocytosis or upregulated expression of scavenger receptors, thereby growing the uptake of exogenous exosomes [32]. As well as, NFAT5 particularly overexpressed stone mice and miR-93-3p antagomir intravenously injection stone mice fashions had been established. M1 polarization, METs formation, and NIK/NF-ΚB2 pathway had been attenuated by NFAT5 overexpression or antagomir (Fig. 6D-E). Histological analyses utilizing HE and Von Kossa staining demonstrated decreased kidney harm and stone formation following NFAT5 overexpression or miR-93-3p inhibition. AAV-NFAT5 or antagomir decreased NIK expression and elevated NFAT5 and p-AKT1 ranges, and METs co-labelled with citH3, the marker of METs, and F4/80 can be suppressed (Fig. 6F-G). These outcomes revealed that exosomal miR-93-3p modulates the NFAT5/AKT1/NIK/NF-κB2 axis in vivo, contributing to renal irritation and harm related to kidney stones.
METs and M1 polarization fashioned a constructive suggestions loop in kidney harm
To additional elucidate the vital position of NIK/NF-κB2 and METs in CaOx-exo-induced kidney harm, we used METs inhibitor CI-amidine or NIK inhibitor NIK SMI1 to suppress METs manufacturing and NIK/NF-κB2 pathway in mice, respectively. As anticipated, each remedies attenuated pathological modifications, together with renal tubule lumen dilation, crystals deposition, M1 polarization, and the presence of citH3 and F4/80-labeled METs (Fig. 7A-B). Western blot evaluation confirmed the decreased expression of iNOS and citH3 proteins within the kidney tissues of handled mice, aligning with staining outcomes (Fig. 7C-D). Moreover, ionomycin, an activator of extracellular traps, considerably elevated METs formation, in addition to the co-localization of citH3 and MMP12 in macrophages. It additionally enhanced M1 polarization in murine bone marrow-derived macrophages. Nonetheless, each CI-amidine and NIK SMI1 successfully mitigated these results (Fig. 7E-F). In every group of macrophages, we evaluated the expression ranges of citH3 and iNOS. Western blot evaluation confirmed that each inhibitors successfully inhibited the upregulation of citH3 and iNOS, in addition to M1 polarization by CaOx and Ionomycin (Fig. 7G-H). In conclusion, pro-inflammatory macrophages and their distinct modes of cell loss of life mutually amplify one another, making a constructive suggestions loop that exacerbates kidney harm.
METs and M1 polarization fashioned a constructive suggestions loop in kidney harm. A–B Consultant photos and quantitative evaluation HE, Von Kosaa, and IF photos displaying the expression of iNOS+, and citH3 + F4/80 + areas. Scale bar = 20 μm. C–D Western blot bands and quantitative protein expression ranges of iNOS and citH3 in kidney tissues from 4 teams of mice. E–F citH3 + F4/80+, iNOS + cells and METs formation had been detected by IF after pre-treatment of BMDMs with CI-amidine, NIK SMI1, or Ionomycin. The outcomes had been quantified. Scale bar = 20 μm. G–H Western blot bands and quantitative protein expression ranges of iNOS and citH3 in BMDMs in 6 teams. Knowledge are proven as imply ± SEM. Two-way ANOVA with Tukey’s a number of comparisons check.*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
miR-93-3p is particularly down-regulated in TCMK-1 cells by the grasp transcription issue complicated CREB1/CRTC2
To discover the mechanism of by which CaOx stimulated the elevated expression of miR-93-3p in TCMK-1 cells, we searched the precise transcription components of mmu-miR-93-3p within the public on-line database TransmiR (v3.0,cuilab.cn/transmir). Screening for transcription components with Stage 1 proof particularly expressed within the kidney, we discovered that CRTC2 is very kidney-specific (Desk S1). CREB1, a coactivator of CRTC2, was additionally recognized as a candidate. Subsequently, we centered on the regulation of miR-93-3p by CREB1/CRTC2 complicated. Intraperitoneal injection of 1,25(OH)2D3 is usually used to ascertain nephrolithiasis fashions in mice [33]. Evaluation of ChIP-seq information from GSE206777 revealed that each p-CREB1 and CRTC2 had been enriched upstream of the miR-93 host gene (miR-93 HG) promoter, with CRTC2 displaying vital enrichment underneath 1,25(OH)₂D₃ stimulation (Fig. 8A). This implies that the transcription coactivator CRTC2 could also be the principle issue answerable for the elevated miR-93-3p expression. CHIP assay confirmed that CRTC2, however not p-CREB1, regulated miR-93-3p expression after CaOx stimulation in TCMK-1 cells (Fig. 8B-C). Electrophoretic mobility shift assay (EMSA) was carried out to additional reveal the precise binding area of CREB1/CRTC2 to the miR-93 HG promoter. Binding to the miR-93 HG promoter was noticed solely in protein extracts from cells stably expressing CREB1 however not with extracts from cells stably expressing CRTC2, and specificity was confirmed by the decreased binding within the presence of aggressive probes (Fig. 8D). That is in keeping with the everyday construction and performance of the CREB1/CRTC2 complicated. Subsequently, we hypothesized that CREB1 instantly binds to the promoter of miR-93 HG, however its enrichment stays fixed no matter stimulation. In distinction, CRTC2 binds not directly to the promoter area by way of CREB1, and is considerably enriched throughout stimulation, functioning because the dominant transcription issue (Fig. 8G). TransmiR offered the putative motif emblem for CREB1, highlighting two potential binding websites on the promoter area (Fig. 8E). CHIP-qPCR revealed that the binding website 1(-243 to -238 bp) was answerable for CREB1/CRTC2-mediated miR-93 HG promoter exercise (Fig. 8F). Luciferase reporter assays in 293T cells confirmed that transfection of CREB1 or CRTC2 plasmids considerably elevated promoter exercise, with CRTC2 demonstrating a stronger impact. Nonetheless, the transcriptional impact of CREB1 or CRTC2 on mutant miR-93 HG was considerably decreased. For miR-93 HG + CRTC2 + shCREB1 group, the luciferase exercise was not solely decrease than that of miR-93 HG + CREB1 + shCRTC2 group but additionally extra considerably decrease than that of mir-93 HG + CRTC2 group (Fig. 8H). These outcomes revealed that CREB1/CRTC2 is a selected transcriptional activator complicated of miR-93 HG. Whereas CREB1 is important for transcription activation, CRTC2 performs a predominant position in enhancing transcription.
miR-93-3p is particularly down-regulated in TCMK-1 cells by the grasp transcription issue complicated CREB1/CRTC2. A CHIP-seq of 1,25(OH)2D3 modelled C57BL/6J mice in GSE206777. Each p-CREB1 and CRTC2 had been enriched on the upstream of promoter of miR-93 host gene (miR-93 HG). B–C Enrichment of CREB1 and CRTC2 on miR-93 HG. D Electrophoretic mobility shift assay (EMSA) for the evaluation of CREB1 and CRTC2 binding on miR-93 HG promoter area. E–F Chip-qPCR assays of CREB1 within the promoter area of miR-93 HG in TCMK-1 cells. G A schematic diagram reveals the construction of the luciferase reporter plasmid. Luciferase exercise was enhanced when the CREB1/CRTC2 complicated was elevated. H Luciferase assay confirmed the impact on the luciferase exercise of cells when transfected with completely different plasmids. Knowledge are proven as imply ± SEM. P-values had been calculated from two-tailed impartial t-tests. One-way ANOVA with Tukey’s a number of comparisons check.*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.