Apt19s-EV acknowledge and recruit O-BMSCs particularly in vitro
The scarcity of BMSCs at injured websites was the primary purpose for poor therapeutic of bone fractures and defects in senescent people. Exogenous BMSCs implantation remedy might convey varied issues, together with immunogenicity, tumorigenicity, and unpredictable survival charges, which restrict its additional medical software and translation [28, 29]. Subsequently, recruiting endogenous stem cells to bone fracture or defect websites has been thought of an efficient technique for addressing these points [30]. Aptamers, small molecular synthetic nucleic acids, can recruit and bind with particular cell sorts, and have been utilized in tissue engineering attributable to their benefits together with low immunogenicity, chemical stability, simple synthesis and modification, and low value [31,32,33]. Apt19s, a 49-base single-stranded DNA sequence, has been extensively utilized for the modification of biomaterial scaffolds to seize endogenous BMSCs in situ, thereby enhancing bone regeneration [34,35,36]. Our earlier research additionally demonstrated that Apt19s-functionalized 3D-printed MBG scaffolds might recruit endogenous BMSCs to additional promote bone regeneration through the SDF-1/CXCR4 and MAPK signaling pathways [37]. In comparison with scaffolds, EV as nanocarriers have particular benefits resembling low immunogenicity or toxicity, simple modification, and a comparatively wealthy content material of drugs. Moreover, the research cited above had been all primarily based on Y-BMSCs, however the senescent microenvironment would impede endogenous stem cell recruitment to the injured websites. Subsequently, the feasibility of establishing of Apt19s-EV and their results on O-BMSCs recruitment and subsequent bone restore require additional elucidation.
In view of those factors, Apt19s-EV was constructed to seize and recruit O-BMSCs. Briefly, the aldehyde-modifed Apt19s was reacted with amino teams on the EV membrane at 4℃ in a single day to assemble Apt19s-EV, together with aptamer random sequence engineered EV (Apt-Rd-EV) and EV as controls (Fig. 1A). Circulation cytometry evaluation demonstrated that the fluorescence depth of Cy5-labeled Apt19s-EV was considerably better than that of EV, indicating the profitable conjugation of Apt19s with EV (Fig. 1B). TEM photographs confirmed that Apt19s-EV, Apt-Rd-EV and EV had been all 30–220 nm spherical, membrane-bound vesicles (Fig. 1C). Western blot evaluation revealed that the markers together with Alix, CD63 and CD81 had been positively expressed in Apt19s-EV, Apt-Rd-EV and EV (Fig. 1D). NTA evaluation demonstrated that the very best peak of Apt19s-EV, Apt-Rd-EV and EV was round 150 nm (Fig. 1E). The outcomes additionally indicated that there have been no vital variations within the focus of Apt19s-EV, Apt-Rd-EV and EV (Supplementary Fig. 1A). DLS outcomes confirmed that the diameter of Apt19s-EV and Apt-Rd-EV had been barely bigger than that of the EV (Supplementary Fig. 1B-D). All these outcomes confirmed that the Apt19s-EV was constructed efficiently.
The precise recognition and binding capacity of Apt19s-EV to stem cells was verified by cell immunofluorescence. The Apt19s (Cy5, pink)-engineered EV (PKH26, inexperienced) was co-incubated with O-BMSCs, chondrocytes, fibroblasts and EPCs, with Apt-Rd-EV and EV as management teams. Clearly, no pink fluorescence was detected in three teams of chondrocytes, fibroblasts and EPCs and the O-BMSCs in EV and Apt-Rd-EV group, whereas O-BMSCs confirmed distinct Cy5 fluorescence labeling within the Apt19s-EV group, indicating that Apt19s can selectively bind to O-BMSCs (Fig. 1F-G and supplementary Fig. 2A-D). As well as, the inexperienced fluorescence indicators of Apt19s-EV had been considerably better in O-BMSCs than these within the Apt-Rd-EV and EV teams (Fig. 1F, H). Nevertheless, no vital modifications in inexperienced fluorescence indicators had been noticed among the many three teams in chondrocytes, fibroblasts and EPCs (Fig. 1G, I and supplementary Fig. 2A-D). These outcomes demonstrated that Apt19s might effectively facilitate the uptake of EV by O-BMSCs. The wound therapeutic and transwell assays had been additionally carried out to analyze the recruitment impact of Apt19s-EV on O-BMSCs. The fluorescence photographs confirmed that the variety of migrated cells within the Apt19s-EV group was considerably greater than these within the different two teams (Fig. 1J, L). As well as, the Apt19s-EV recruited extra O-BMSCs to the decrease floor of the chamber than the opposite two teams (Fig. 1Ok, M). In abstract, these knowledge confirmed that Apt19s-EV might particularly acknowledge and effectively recruit O-BMSCs in vitro.
Development, characterization and organic features of Apt19s-EV. (A) Schematic illustration of Apt19s-EV development. (B) The Cy5 fluorescence depth of Apt19s-EV and EV. (C) The TEM photographs of EV, Apt-Rd-EV and Apt19s-EV. (D) Western blot of the markers of EVs together with Alix, CD63 and CD81 in EV, Apt-Rd-EV and Apt19s-EV. (E) Particle dimension distribution of EV, Apt-Rd-EV and Apt19s-EV. (F-G) The pictures of EV, Apt-Rd-EV and Apt19s-EV internalization by O-BMSCs and chondrocytes. (EV, Apt-Rd-EV and Apt19s-EV labeled with PKH67 (inexperienced); Apt19s labeled with Cy5 (Pink)). (H-I) The quantitative evaluation of the fluorescence depth of EV, Apt-Rd-EV and Apt19s-EV in O-BMSCs and chondrocytes. (J-M) Analysis of migratory capacity and quantitative evaluation of O-BMSCs cultured in EV, Apt-Rd-EV and Apt19s-EV by wound therapeutic (J, L) and Transwell assay (Ok, M). Knowledge represented as imply ± SD (n = 3). The importance of the information was calculated by the one-way ANOVA. (Y-rat, Younger rats; Y-BMSCs, BMSCs derived from younger rats; O-BMSCs, BMSCs derived from previous rats; Apt-Rd-EV, Aptamer random sequence engineered extracellular vesicles; *signifies vital variations in contrast with EV; # signifies vital variations in contrast with Apt-Rd-EV, p < 0.05; ns, no significance; Scale bar, 100 μm.)
miR-376b-5p was chosen because the candidate for establishing engineered EV
Within the senescent microenvironment, the decline in osteogenic differentiation of BMSCs was one more reason for compromised bone restore. Epigenetic modifications had been the vital hallmarks of BMSCs senescence, considerably affecting each the senescent and osteogenic processes [38]. Particularly, miRNAs have emerged as key diagnostic and therapeutic biomarkers for BMSCs senescence and osteogenic differentiation by binding to the three’-UTR of goal mRNAs [39]. For instance, miR-188 led to an imbalance between osteogenesis and adipogenesis, in the end leading to bone loss and fats accumulation in senescent mice [40]; Overexpression of miR-384-5p inhibits osteogenic differentiation and accelerates senescence of BMSCs by concentrating on Gli2, contributing to age-related bone loss [41]. Just lately, EV have been proven to successfully enhance bone regeneration and forestall osteoporotic bone loss in bone senescent-related ailments by means of delivering miRNAs [42]. Lu et al. confirmed that miR-29a overexpressing engineered EV might promote osteogenesis and angiogenesis, serving as a possible biomarker for osteoporosis [43]. Subsequently, choosing practical miRNAs from O-BMSCs and Y-BMSCs as candidates for establishing engineered EV was anticipated to reverse or alleviate the development of senescent bone-related ailments.
To display screen practical miRNAs, Y-BMSCs and O-BMSCs had been remoted and cultured for RNA sequencing (Fig. 2A). The highest 20 downregulated miRNAs in O-BMSCs had been chosen for additional affirmation by qRT-PCR (Fig. 2B). After validation, the expression of miR-6321, miR-540-3p, miR-6331, miR-344b-5p, miR-673-3p, miR-341, miR-434-5p, miR-3854-3p, miR-1188-5p, miR-376c-5p and miR-376b-5p in O-BMSCs was considerably decreased in comparison with these in Y-BMSCs (Supplementary Fig. 3A). Of notice, the expression of miR-540-3p, miR-434-5p and miR-376b-5p confirmed the best variations between Y-BMSCs and O-BMSCs (Fig. 2C). A earlier research demonstrated that miR-540-3p was concerned within the immunoregulatory results mediated by extracellular vesicles derived from BMSCs stimulated by indoleamine 2,3-dioxygenase (IDO) [44]. Moreover, miR-376b-5p was linked to vascular formation and calcification processes [45, 46]. The opposite differential miRNAs haven’t been reported to be associated to osteogenesis, angiogenesis or senescence. Subsequently, contemplating the above outcomes and former literature, three candidate miRNAs (miR-540-3p, miR-376b-5p and miR-434-5p) had been chosen for the next experiments.
O-BMSCs had been then transfected with miRNA mimics to additional examine the results of the above miRNAs on osteogenesis and senescence. As indicated in Supplementary Fig. 3B-D, the expression of miR-540-3p, miR-376b-5p and miR-434-5p was all extremely upregulated in O-BMSCs after miRNA mimics transfection, demonstrating profitable transfection of the three miRNAs. ALP staining demonstrated that the osteogenic differentiation capability was elevated after miR-540-3p and miR-376b-5p transfection, whereas an reverse development was noticed within the miR-434-5p mimic group (Fig. 2D). As well as, the variety of SA-β-gal constructive cells was considerably decreased in O-BMSCs transfected with miR-540-3p, miR-376b-5p and miR-434-5p mimics in comparison with these transfected with mimics NC (Fig. 2E). The gene expression of osteogenic differentiation markers (ALP, BMP-2, Col-1 and OCN) and senescent markers (SATB2, p53, p21 and p16) was additional explored after 3-day and 7-day miRNA mimics transfection by qRT-PCR. After 3-day transfection, the expression of ALP and OCN was considerably elevated in all three miRNA-transfected teams (Fig. 2F and I). The expression of BMP-2 and Col-1 was extremely upregulated in miR-376b-5p and miR-434-5p transfected cells, whereas these two genes expression had been downregulated in miR-540-3p transfected cells (Fig. 2G-H). The expression of SATB2 confirmed minimal vital modifications in all three transfected teams (Fig. 2J). The expression of senescent markers p53, p21 and p16 was considerably decreased in all three transfected teams, anticipate for p21 expression within the miR-434-5p transfected group (Fig. 2Ok-M). After 7-day transfection, the expression of all these osteogenic differentiation markers was elevated, anticipate for ALP and BMP-2 within the miR-434-5p transfected group (Supplementary Fig. 3E-H). Of notice, the expression of SATB2 was considerably upregulated after 7-day miR-376b-5p transfection (Supplementary Fig. 3I). The expression of p53, p21 and p16 had been all downregulated after miR-540-3p, miR-376b-5p and miR-434-5p transfection, anticipate for p53 in miR-540-3p mimic group (Supplementary Fig. 3J-L). Moreover, western blot assay was carried out to additional validate the qRT-PCR outcomes. As indicated in Fig. 2N and Supplementary Fig. 3M, the protein expression of osteogenic markers (Col-1, BSP, OCN) was all extremely expressed after 3-day miR-376b-5p transfection. After 3-day transfection, the protein expression of SATB2 was considerably upregulated within the miR-540-3p and miR-376b-5p transfected teams (Fig. 2O and Supplementary Fig. 3N). The protein expression of senescent markers (p53, p21, p16) was all considerably downregulated after transfection, anticipate for p21 in miR-376b-5p transfected cells (Fig. 2O and Supplementary Fig. 3N). After 7-day transfection, the expression of Col-1, BSP and OCN was notably elevated, apart from BSP within the miR-434-5p mimic group and OCN within the miR-540-3p and miR-434-5p mimic teams (Supplementary Fig. 3O-P). The senescent markers together with SATB2, p53, p21 and p16 confirmed nice significance, anticipate for p16 within the miR-540-3p and miR-434-5p mimic teams (Supplementary Fig. 3Q-R). Moreover, immunofluorescence staining demonstrated that osteogeinc proteins (BMP-2 and Col-1) exhibited the very best expression within the miR-376b-5p transfection group (Fig. 2P-Q). The above outcomes demonstrated that miR-376b-5p and miR-434-5p had related results on the senescent phenotype together with gene and protein expression. Nevertheless, ALP staining indicated that miR-376b-5p might improve osteogenic differentiation, whereas miR-434-5p decreased osteogenic differentiation. The qRT-PCR and WB outcomes additionally demonstrated that miR-376b-5p promoted osteogenesis extra successfully than miR-434-5p. In addition to, the immunofluorescence staining indicated that the protein expression of BMP-2 and Col-1 was highest after miR-376b-5p mimic transfection. Regardless of related results on senescence, miR-376b-5p confirmed better potential for selling osteogenesis in O-BMSCs.
EV play very important roles in varied bone-related ailments by delivering miRNAs cargoes inherited from parental cells [47]. In the meantime, modifications in EV miRNAs can affect intercellular communication, which is a key function of senescent bone-related ailments. Fulzele et al. detected considerably elevated EV miR-34a-5p derived from skeletal muscle and serum of aged mice, which instantly accelerated the getting old strategy of BMSCs [48]. Additionally, a earlier research indicated that exosomal miR-31a-5p derived from senescent BMSCs was considerably greater than that derived from younger BMSCs, which can be an vital issue for osteoclast absorption and a possible goal for treating aging-related osteoporosis [49]. Within the current research, EV remoted from O-BMSCs and Y-BMSCs (O-EV and Y-EV) had been collected to additional examine whether or not the expression of the above three miRNAs was decreased in O-EV. TEM, WB NTA and DLS evaluation had been carried out to determine the Y-EV and O-EV (Supplementary Fig. 4A-D). As well as, there was no vital distinction within the focus of Y-EV and O-EV (Supplementary Fig. 4E). Moreover, the outcomes demonstrated the expression of miR-540-3p, miR-434-5p and miR-376b-5p in O-EV was considerably decreased in comparison with that in Y-EV (Supplementary Fig. 4F). Of notice, the expression of miR-376b-5p in O-EV confirmed the best distinction amongst these three miRNAs. Subsequently, contemplating its particular features and the best differential expression in O-BMSCs and O-EV, miR-376b-5p was chosen because the candidate to additional assemble engineered EV for the next experiments.
miR-376b-5p was chosen because the candidate for establishing engineered EV. (A) Schematic illustration of cell isolation and RNA sequencing of Y-BMSCs and O-BMSCs. (B) The heatmap of high 20 down-regulated miRNAs of O-BMSCs and Y-BMSCs. (C) The expression of miR-540-3p, miR-376b-5p and miR-434-5p by qRT-PCR. (D-E) The ALP (D) and SA-β-gal (E) staining of O-BMSCs after miR-540-3p, miR-376b-5p and miR-434-5p mimics transfection. (F-M) The three-day osteogenic (ALP, BMP-2, Col-1 and OCN) and senescent (SATB2, p16, p21 and p53) genes expression of O-BMSCs after miR-540-3p, miR-376b-5p and miR-434-5p mimics transfection. (N-O) The three-day osteogenic (Col-1, BSP and OCN) and senescent (SATB2, p16, p21 and p53) proteins ranges of O-BMSCs after miR-540-3p, miR-376b-5p and miR-434-5p mimics transfection. (P-Q) The immunofluorescence staining of osteogenic-related protein BMP-2 and Col-1 of O-BMSCs after miR-540-3p, miR-376b-5p and miR-434-5p mimics transfection. Knowledge represented as imply ± SD (n = 3). The importance of the information was calculated by the one-way ANOVA (540 mimics, miR-540-3p mimics; 376b mimics, miR-376b-5p mimics; 434 mimics, miR-434-5p mimics; *p < 0.05, **p < 0.01, ***p < 0.001. Scale bar: 100 μm)
376b-EV promotes osteogenesis and alleviate senescence of O-BMSCs
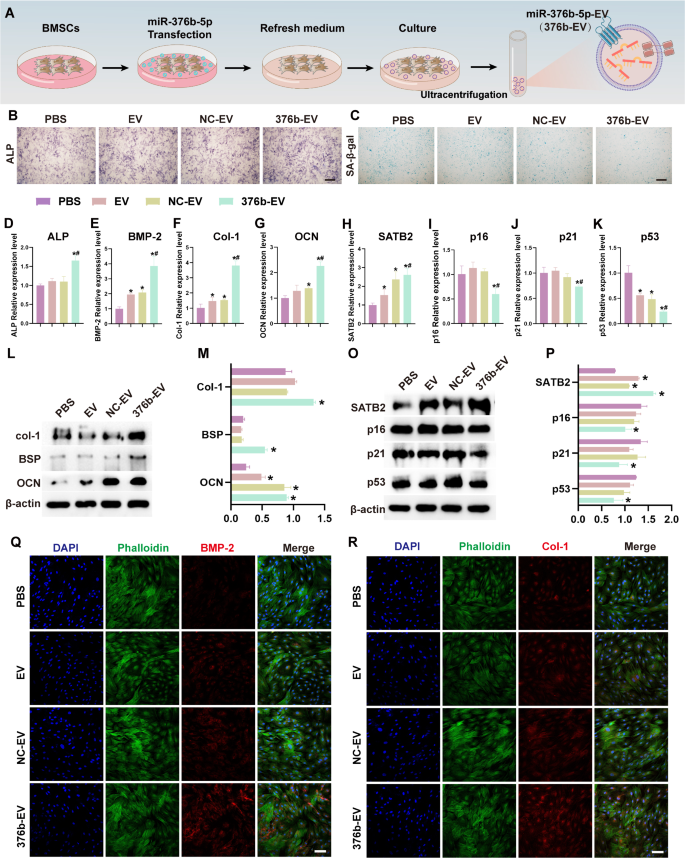
After choosing miR-376b-5p, the cell transfection methodology was utilized to assemble miR-376b-5p engineered EV (376b-EV) to additional examine their results on osteogenesis and senescence of O-BMSCs (Fig. 3A). Notably, the expression of miR-376b-5p was extremely elevated within the 376b-EV group in comparison with the EV or miR-376b-5p destructive management group (NC-EV) (Supplementary Fig. 5A). Gradient concentrations (50 µg/ml, 100 µg/ml, 200 µg/ml) of various EV had been co-cultured with O-BMSCs to display screen for the optimum focus by CCK-8 assay. The outcomes indicated that each one the three EV exhibited a pro-proliferation impact, with the very best OD worth at 100 µg/ml (Supplementary Fig. 5B). As well as, 376b-EV promoted the proliferation of O-BMSCs after 3-day and 5-day incubation (Supplementary Fig. 5C). Subsequently, 100 µg/ml was chosen as essentially the most acceptable focus for the next experiments. The expression of miR-376b-5p in O-BMSCs was notably elevated after therapy with 376b-EV in comparison with cells handled with EV or NC-EV (Supplementary Fig. 5D). The fluorescence photographs illustrated that the PKH67-labeled extracellular vesicles had been internalized and distributed across the cell nucleus of O-BMSCs (Supplementary Fig. 5E). Collectively, these outcomes demonstrated that 376b-EV was efficiently constructed and internalized into O-BMSCs, delivering the elevated miR-376b-5p cargo.
Then, the organic results of 376b-EV on O-BMSCs was investigated. As proven in Fig. 3B, ALP staining indicated that 376b-EV might improve the osteogenic differentiation of O-BMSCs. In distinction, the variety of SA-β-gal-positive cells was considerably decreased after therapy with 376b-EV (Fig. 3C). qRT-PCR demonstrated that 376b-EV might considerably enhance the expression of osteogenic markers (ALP, BMP-2, Col-1 and OCN) and the senescent marker (SATB2) in O-BMSCs in comparison with cells handled with PBS, NC-EV or EV (Fig. 3D-H). As well as, the expression of senescent markers (p53, p21 and p16) was additionally notably decreased after therapy with 376b-EV (Fig. 3I-Ok). Moreover, the western blot outcomes additionally demonstrated that the protein expression of Col-1, BSP and OCN was considerably elevated by 376b-EV stimulation (Fig. 3L-M). As well as, the senescent marker, SATB2, was additionally elevated after stimulation with 376b-EV (Fig. 3O-P). Conversely, different senescent markers together with p53, p21 and p16 had been considerably down-regulated after co-culturing with 376b-EV (Fig. 3O-P). Moreover, the immunofluorescence staining demonstrated that the expression of osteogenic proteins (BMP-2 and Col-1) was elevated after therapy with 376b-EV (Fig. 3Q-R). Collectively, our outcomes point out that 376b-EV might enhance proliferation, osteogenic differentiation capability, and alleviate the senescent-related phenotype of O-BMSCs by delivering practical miR-376b-5p cargo.
Just lately, EV particularly these derived from younger people have exhibited distinctive benefits in bone-related degenerative ailments. For instance, EV remoted from neonatal umbilical cords rejuvenate aged BMSCs and gradual age-related degeneration by delivering PCNA [50]. One other research revealed that stem cell-derived EV might reverse BMSCs senescence and age-related osteogenic dysfunction, thereby stopping age-related bone loss [51]. In our current research, EV remoted from Y-BMSCs had been proven to control osteogenesis and senescence. On this foundation, 376b-EV constructed from Y-BMSCs transfected with mimics combines some great benefits of Y-EV and miR-376b-5p. Subsequently, the engineered EV, 376b-EV, is anticipated to reverse or alleviate the development of senescent bone-related ailments. Nevertheless, the potential mechanism underlying 376b-EV-mediated osteogenesis and senescence nonetheless requires additional elucidation.
376b-EV promotes osteogenesis and alleviate senescence of O-BMSCs. (A) Schematic illustration of miR-376b-5p overexpressing engineered extracellular vesicles (376b-EV) development. (B-C) The ALP (B) and SA-β-gal (C) staining of O-BMSCs cultured with 376b-EV. (D-Ok) The osteogenic (ALP, BMP-2, Col-1 and OCN) and senescent (SATB2, p16, p21 and p53) of O-BMSCs cultured with 376b-EV. (L-P) The osteogenic (L-M) and senescent (O-P) proteins ranges and its quantitative statistics of O-BMSCs cultured with 376b-EV. (Q-R) The immunofluorescence staining of osteogenic-related protein BMP-2 and Col-1 of O-BMSCs cultured with 376b-EV. Knowledge represented as imply ± SD (n = 3). The importance of the information was calculated by the one-way ANOVA (NC-EV, miR-376b-5p destructive management engineered extracellular vesicles; 376b-EV, miR-376b-5p overexpressing engineered extracellular vesicles; *p < 0.05, **p < 0.01, ***p < 0.001. Scale bar: 100 μm)
376b-EV mediates osteogenesis and senescence of O-BMSCs by means of concentrating on Camsap1
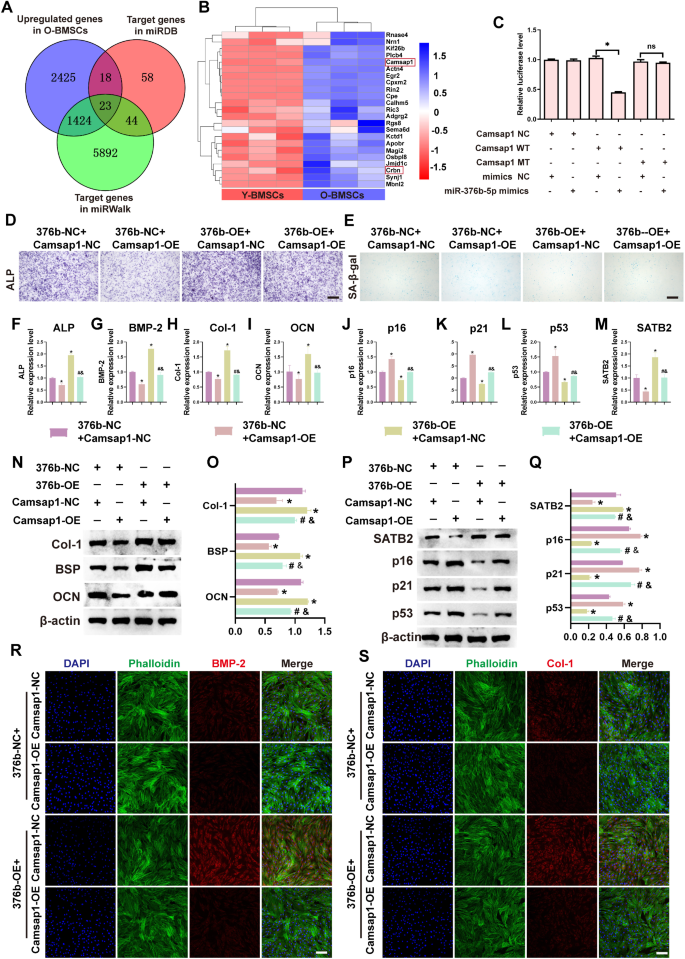
miRNAs, as non-coding RNAs, normally perform by binding to the three’UTR websites of goal genes to inhibit their expression [52, 53]. To additional elucidate the mechanism of 376b-EV-mediated osteogenesis and senescence, miRDB and miRwalk had been used to foretell the potential targets of miR-376b-5p. As indicated in Figs. 4A-B and 23 potential targets had been recognized after intersecting the targets predicted by miRDB and miRwalk with the upregulated differential mRNAs in O-BMSCs from RNA sequencing. Of notice, Camsap1 and Crbn had been chosen because the targeted amongst these focused genes. The earlier research has demonstrated that Camsap1 instantly regulated by miR-126 was important for bone improvement, fracture therapeutic, and bone tissue engineering [54]. As well as, Crbn has been proven to successfully regulate the event of osteoporosis and is taken into account a possible therapy choice for diabetes-induced osteolytic bone illness [55]. Nevertheless, the expression and detailed regulatory results of those goal genes on O-BMSCs wanted additional clarification. First, the expression of Camsap1 and Crbn was greater in O-BMSCs in comparison with Y-BMSCs, per the outcomes from RNA sequencing (Supplementary Fig. 6A). As well as, the expression of Camsap1, however not Crbn, was notably decreased after O-BMSCs transfected with miR-376b-5p mimics (Supplementary Fig. 6B). Moreover, the expression of Camsap1 in O-BMSCs was additionally decreased after therapy with 376b-EV (Supplementary Fig. 6C). The luciferase assay demonstrated that the luciferase exercise of the Camsap1-WT reporters, however not the Camsap1-MT reporters, was repressed after miR-376b-5p mimics transfection (Fig. 4C). Based mostly on these outcomes, Camsap1 was chosen because the potential goal gene of miR-376b-5p to analyze the underlying regulatory mechanism.
To additional confirm whether or not miR-376b-5p regulates osteogenesis and rescues senescence in O-BMSCs by means of concentrating on Camsap1, Camsap1 overexpression plasmids (Camsap1-OE) and destructive management plasmids (NC) had been constructed and co-transfected with miR-376b-5p mimics and NC (376b-OE/NC). As proven in Fig. 4D, ALP staining demonstrated that the osteogenesis enhanced by 376b-OE was considerably decreased after co-transfection with Camsap1-OE plasmids. In distinction, overexpression of miR-376b-5p decreased the variety of SA-β-gal-positive stained cells; nevertheless, these results had been considerably rescued when the cells had been co-transfected with Camsap1-OE plasmids (Fig. 4E). As well as, qRT-PCR evaluation demonstrated that 376b-OE-upregulated osteogenic markers (ALP, BMP-2, Col-1 and OCN) and the senescent marker (SATB2), and these results had been partially blocked by Camsap1-OE plasmids (Fig. 4F-J). The opposite 376b-OE-downregulated senescent markers (p53, p21 and p16) had been additionally rescued to close baseline ranges when the cells had been co-transfected with Camsap1-OE plasmids (Fig. 4Ok-M). Moreover, the protein expression of osteogenic and senescent markers displayed an analogous development to the qRT-PCR outcomes. The outcomes confirmed that the osteogenic markers (Col-1, BSP and OCN) and the senescent marker (SATB2) had been considerably elevated after 376b-OE transfection, and the upregulated ranges had been decreased after co-transfection with Camsap1-OE plasmids (Fig. 4N-Q). Furthermore, the expression of different senescent markers (p16, p21, p53) in O-BMSCs was decreased by 376b-OE transfection and elevated by co-transfection with Camsap1-OE plasmids (Fig. 4P-Q). The cell immunofluorescence staining demonstrated that the 376b-OE-induced upregulation of osteogenic-related proteins ( BMP-2 and Col-1) was compromised when the cells had been co-transfected with 376b-OE and Camsap1-OE plasmids (Fig. 4R-S). Collectively, these knowledge indicated that 376b-EV enhance osteogenesis and alleviate senescence of O-BMSCs by delivering miR-376b-5p, which instantly targets the three’-UTR areas of Camsap1.
376b-EV mediates osteogenesis and senescence of O-BMSCs by means of concentrating on Camsap1. (A) Goal genes screening. (B) The heatmap of goal genes. (C) The luciferase exercise detection after transfecting with Camsap1 reporter or Camsap1 MT reporter and miR-376b-5p mimics or NC. (D-E) The ALP staining (D) and SA-β-gal staining (E) after co-transfecting 376b-OE/NC and Camsap1-OE/NC. (F-M) The osteogenic (ALP, BMP-2, Col-1 and OCN) and senescent (SATB2, p16, p21 and p53) of O-BMSCs after co-transfecting 376b-OE/NC and Camsap1-OE/NC. (N-Q) The osteogenic (N-O) and senescent (P-Q) proteins ranges and its quantitative statistics of O-BMSCs after co-transfecting 376b-OE/NC and Camsap1-OE/NC. (R-S) The immunofluorescence staining of osteogenic-related protein BMP-2 and Col-1 of O-BMSCs after co-transfecting 376b-OE/NC and Camsap1-OE/NC. Knowledge represented as imply ± SD (n = 3). The importance of the information was calculated by the one-way ANOVA. (376b-NC, miR-376b-5p destructive management transfection; 376b-OE, miR-376b-5p mimics transfection; Camsap1-NC, Camsap1 destructive management transfection; Camsap1-OE, Camsap1 overexpression plasmids transfection; *signifies vital variations in contrast with 376b-NC + Camsap1-NC group; #signifies vital variations in contrast with 376b-NC + Camsap1-OE group; &signifies vital variations in contrast with 376b-OE + Camsap1-NC group; p < 0.05, scale bar: 100 μm)
Apt-376b-EV@GelMA promotes cell recruitment and osteogenesis of O-BMSCs sequentially in vitro
To combine some great benefits of Apt19s and miR-376b-5p, a dual-engineered EV (Apt-376b-EV) with sequential cell recruitment and osteogenic features was constructed by means of miR-376b-5p transfection and Apt19s incubation (Fig. 5A). EV was simply cleared and quickly launched, which is a significant purpose for the low effectivity of bone defect restore, particularly in getting old microenvironments. Subsequently, appropriate scaffolds for loading EV are important for efficient bone restore. Gelatin methacryloyl (GelMA) hydrogel has been extensively used for varied biomedical functions attributable to its glorious organic properties and versatile software in several medical situations [56,57,58]. Current research have additionally demonstrated some great benefits of GelMA hydrogel as a scaffold for drug or EV supply in bone tissue engineering [59, 60]. Subsequently, GelMA hydrogel was chosen to encapsulate Apt-376b-EV to additional examine its results on bone regeneration each in vitro and in vivo.
Fluorescence photographs confirmed that Cy5-labeled Apt-376b-EVwere uniformly distributed within the GelMA hydrogel (Fig. 5B). As well as, the Apt-376b-EV was launched stably from the Apt-376b-EV@GelMA scaffold inside two weeks (Fig. 5C). These outcomes confirmed that the Apt-376b-EV@GelMA sustained supply scaffold was constructed efficiently. Fluorescent photographs confirmed that the cells exhibited a broad and flat morphology with prolonged tentacles (Supplementary Fig. 7A-B). Transwell chambers had been used to guage the results of cell recruitment, osteogenesis and senescence of the Apt-376b-EV@GelMA scaffold on O-BMSCs (Fig. 5D-E). The transwell assay demonstrated that the Apt-376b-EV@GelMA scaffold recruited extra O-BMSCs, which migrated to the decrease floor of the chamber in comparison with the opposite teams (Fig. 5F). SA-β-gal staining additionally demonstrated that the Apt-376b-EV@GelMA scaffold might attenuate the senescence of O-BMSCs (Fig. 5G). Moreover, ALP staining demonstrated that the Apt-376b-EV@GelMA scaffold might promote osteogenic differentiation of O-BMSCs in vitro (Fig. 5H).
Within the current research, we used GelMA hydrogel as a service for Apt-376b-EV supply attributable to its glorious organic properties and versatile software. GelMA hydrogel possesses nice biocompability and may mimic the pure ECM for cell adhesion and development as a result of presence of cell-attaching and matrix metalloproteinase responsive peptide motifs. Extra importantly, the Apt-376b-EV@GelMA hydrogel scaffold with sequential cell recruitment and osteogenic differentiation exhibited vital benefits in getting old microenvironments. Nevertheless, different kinds of biomaterials together with bioactive membranes, porous scaffolds and different composite supplies may be thought of as carriers for EV supply. The strategies of incorporating EV into biomaterials may also present a sustained launch impact. In addition to, direct injection of EV didn’t introduce any extra organic parts, which was extra conducive to additional medical translation. Nevertheless, direct injection of EV could also be shortly cleared and requires excessive focused specificity. In conclusion, totally different EV supply strategies ought to be utilized in line with diverse medical situations, which could possibly be helpful for future medical translation.
Apt-376b-EV@GelMA promotes cell recruitment and osteogenesis of O-BMSCs sequentially in vitro. (A) Schematic illustration of twin engineered extracellular vesicles (Apt-376b-EV) development and Apt-376b-EV@GelMA scaffolds fabrication. (B) The 3D fluorescent picture of Apt-376b-EV@GelMA scaffold noticed by CLSM. (scale bar: 100 μm) (C) The discharge curve of Apt-376b-EV from GelMA scaffold. (D) Schematic illustration of Transwell assay (higher: O-BMSCs; decrease: Apt-376b-EV@GelMA scaffold). (E) Schematic illustration of co-culture system (higher: Apt-376b-EV@GelMA scaffold; decrease: O-BMSCs). (F-H) Analysis of migratory capacity (F), senescence (G) and osteogenesis (H) of O-BMSCs induced by Apt-376b-EV@GelMA scaffolds. (NC-EV, miR-376b-5p destructive management engineered extracellular vesicles; 376b-EV, miR-376b-5p overexpressing engineered extracellular vesicles; Apt-376b-EV, Apt19s and miR-376b-5p overexpressing twin engineered extracellular vesicle; Scale bar, 100 μm.)
Apt-376b-EV@GelMA promotes endogenous stem cells recruitment in vivo
We then constructed SD rat mandibular fracture fashions to guage the stem cell recruitment impact of Apt-376b-EV@GelMA in vivo (Fig. 6A). First, the Apt-376b-EV@GelMA was injected into the bone fracture hole after which crosslinked by gentle irradiation to look at its stem cell recruitment impact. After 2 weeks, the micro-CT reconstruction outcomes demonstrated that solely a small quantity of recent bone had shaped on the junction of the titanium plate (Fig. 6B). The quantitative statistical evaluation demonstrated that the Apt-376b-EV@GelMA group considerably elevated the worth of the BV/TV worth, indicating that Apt-376b-EV@GelMA induced better new bone formation within the fracture hole (Fig. 6C). The immunofluorescence staining was carried out to research stem cell recruitment of Apt-376b-EV@GelMA within the bone fracture space. Of notice, CD90 and CD105 had been chosen as particular stem cell markers. As proven in Fig. 6D, the inexperienced and pink fluorescent areas within the Apt-376b-EV@GelMA group had been considerably extra ample than these within the different teams, indicating that the expression of CD90 and CD105 within the Apt-376b-EV@GelMA group was considerably greater than that within the different teams. Of notice, the quantitative evaluation additionally demonstrated that the fluorescent depth of CD90 and CD105 was considerably elevated within the Apt-376b-EV@GelMA group in comparison with the opposite teams (Fig. 6E-F). Subsequently, the Apt-376b-EV@GelMA scaffold might recruit endogenous stem cells to the bone fracture space, offering a mobile microenvironment conducive to provoke bone restore early.
The earlier research demonstrated that inherent elements might recruit endogenous stem cells to the injured websites after extreme injury [61]. Nevertheless, the senescent microenvironment might intrude with these processes, thereby reducing stem cell homing. Subsequently, the technique of mobilizing endogenous BMSCs to the broken websites is essential for the therapy of bone fractures or defects. Within the current research, the outcomes demonstrated that Apt19s launched from the Apt-376b-EV@GelMA scaffold considerably improved BMSCs recruitment in vivo. Nevertheless, the osteogenic results of Apt-376b-EV@GelMA on bone fractures and defects in vivo wanted additional elucidation.
Apt-376b-EV@GelMA promotes endogenous stem cells recruitment in vivo. (A) Schematic illustration of the applying of Apt-376b-EV@GelMA for mandibular bone fracture in aged rats. (B-C) Micro-CT evaluation of bone formation on the fracture hole (B) and its quantification of bone quantity/whole quantity (BV/TV) (C) after 2-week operation. (D-F) Immunofluorescence staining photographs and quantitative evaluation of CD90-positive and CD105-positive BMSCs recruited by the Apt-376b-EV@GelMA hydrogel in bone fractures of aged rars. Knowledge represented as imply ± SD (n = 6). The importance of the information was calculated by the one-way ANOVA. (NC-EV, miR-376b-5p destructive management engineered extracellular vesicles; 376b-EV, miR-376b-5p overexpressing engineered extracellular vesicles; Apt-376b-EV, Apt19s and miR-376b-5p overexpressing twin engineered extracellular vesicle; *signifies vital variations in contrast with PBS@GelMA group; # signifies vital variations in contrast with EV@GelMA group; & signifies vital variations in contrast with 376b-EV@GelMA group; p < 0.05. Scale bar, 100 μm.)
Apt-376b-EV@GelMA promotes senescent bone fracture therapeutic in vivo
4-week bone fracture specimens had been collected to additional consider the osteogenic results of Apt-376b-EV@GelMA in vivo. The 3D-reconstructed photographs of the 4-week bone fractures confirmed that solely a small quantity of recent bone tissue was shaped within the bone fracture hole of the PBS@GelMA, EV@GelMA and NC-EV@GelMA teams. In distinction, the quantity of recent bone within the 376b-EV@GelMA and Apt-376b-EV@GelMA teams was clearly greater than within the different three teams (Fig. 7A). The micro-CT evaluation additionally demonstrated that the BV/TV values for the 376b-EV@GelMA group had been considerably greater than these for the PBS@GelMA, EV@GelMA and NC-EV@GelMA teams (Fig. 7B), which was associated to the sustained launch of miR-376b-5p from the GelMA scaffold. As well as, the BV/TV worth for the Apt-376b-EV@GelMA group was considerably greater than that for the 376b-EV@GelMA group (Fig. 7B), which can be attributed to the collaborative impact of Apt19s-mediated stem cell homing and miR-376b-5p-mediated osteogenesis. Taken collectively, our outcomes indicated that the Apt-376b-EV launched from GelMA hydrogel had a synergistic impact on selling bone fracture therapeutic.
Histological sections had been stained to additional assess bone therapeutic within the fracture areas. HE and Masson staining confirmed that a considerable amount of fibrous connective tissue with restricted bone formation stuffed the fracture space within the PBS@GelMA, EV@GelMA and NC-EV@GelMA teams. A small quantity of recent bone had grown into the fracture hole within the 376b-EV@GelMA group. Particularly, a considerable amount of full and mature new regenerated bone stuffed the fracture space within the Apt-376b-EV@GelMA group (Fig. 7C-D). The quantitative evaluation additionally demonstrated that the realm proportion and thickness of bone trabeculae within the 376b-EV@GelMA group had been bigger than within the different teams (Fig. 7E-F). To additional affirm that Apt-376b-EV@GelMA promotes bone fracture therapeutic in vivo, immunofluorescence staining for osteogenic markers (OPN, OCN) was carried out. The variety of OPN- and OCN-positive cells within the 376b-EV@GelMA and Apt-376b-EV@GelMA teams was better than within the different three teams (Fig. 7G-I). As well as, the outcomes additionally demonstrated that the Apt-376b-EV@GelMA group exhibited the very best variety of OPN- and OCN-positive cells with the strongest fluorescence, reconfirming the stronger pro-osteogenesis capability of Apt-376b-EV within the senescent microenvironment (Fig. 7G-I).
Apt-376b-EV@GelMA promotes senescent bone fracture therapeutic in vivo. (A-B) Micro-CT evaluation of bone formation on the fracture hole (A) and its quantification of bone quantity/whole quantity (BV/TV) (B) after 4-week operation. (C-F) The bone regeneration analysis of Apt-376b-EV@GelMA after 4 weeks post-implantation by HE (C) and Masson staining (D), and their quantification (E-F). (G-I) Immunofluorescence staining photographs (G) and quantitative evaluation (H-I) of OPN-positive and OCN-positive areas induced by the Apt-376b-EV@GelMA hydrogel in bone fractures of aged rars. Knowledge represented as imply ± SD (n = 6). The importance of the information was calculated by the one-way ANOVA. (NC-EV, miR-376b-5p destructive management engineered extracellular vesicles; 376b-EV, miR-376b-5p overexpressing engineered extracellular vesicles; Apt-376b-EV, Apt19s and miR-376b-5p overexpressing twin engineered extracellular vesicle; T, Trabecula; NB, New bone; M, Muscle; F, Fiber.*signifies vital variations in contrast with PBS@GelMA group; # signifies vital variations in contrast with EV@GelMA group; & signifies vital variations in contrast with 376b-EV@GelMA group; p < 0.05. Scale bar, 100 μm.)
Apt-376b-EV@GelMA enhances senescent vital bone defect restore in vivo
In contrast to linear bone fracture therapeutic, vital bone defects within the senescent microenvironment are more difficult to restore. To adapt to medical situations of bone defects, cylindrical Apt-376b-EV@GelMA scaffolds had been constructed and implanted into vital mandibular bone defects of aged rats to guage their bone restore results (Fig. 8A). The outcomes of micro-CT reconstruction demonstrated that the PBS@GelMA, EV@GelMA and NC-EV@GelMA teams exhibited vital bone loss with out noticeable new bone formation (Fig. 8B). New bone formation on the margin websites of the defects was noticed within the 376b-EV@GelMA group (Fig. 8B). In the meantime, the quantity and thickness of recent bone within the Apt-376b-EV@GelMA group had been considerably greater than within the different teams (Fig. 8B). The micro-CT quantitative outcomes additionally displayed that Apt-376b-EV@GelMA induced the very best new bone parameters (BV/TV) (Fig. 8C). HE and Masson staining clearly confirmed that giant bone defects with out tissue ingrowth had been noticed within the PBS@GelMA group (Fig. 8D-G). As well as, the EV@GelMA and NC-EV@GelMA teams confirmed some muscle and fibrous tissue and restricted new bone on the margins of the defects (Fig. 8D-G). Newly shaped and mature bone was noticed within the 376b-EV@GelMA and Apt-376b-EV@GelMA teams, with the biggest trabecular bone rising into the defects within the Apt-376b-EV@GelMA group (Fig. 8D-G). OPN and OCN secreted by osteoblasts are the vital markers within the course of of recent bone matrix mineralization. Immunofluorescence staining was used to guage the expression of OCN and OPN within the bone defects to evaluate the bone restore results. The PBS@GelMA group exhibited the least expression of OCN and OPN (Fig. 8H-J). As well as, there was no vital distinction within the constructive expression of OPN and OCN cells between the EV@GelMA and NC-EV@GelMA teams (Fig. 8H-J). Greater constructive expression of OPN and OCN cells was noticed within the 376b-EV@GelMA group in comparison with the opposite three teams (Fig. 8H-J). Particularly, there was an apparent enhance within the constructive expression of OPN and OCN within the areas contained in the defects (Fig. 8H-J), indicating that Apt19s and miR-376b-5p synergistically promote the expression of OCN and OPN to facilitate new bone formation within the bone defect areas.
In our current research, bone fractures and bone defects restore had been evaluated at 4 and eight weeks respectively. The outcomes clearly demonstrated that Apt-376b-EV@GelMA confirmed better bone restore in comparison with different teams. Nevertheless, after reviewing the associated literature, it was famous that the statement interval for rat mandibular defects ranged from 6 to 12 weeks [62,63,64,65], whereas the statement interval for rat mandibular fractures ranged from 4 to 7 weeks [66,67,68]. On account of sturdy bone resorption and inflammatory secretion, senescent people exhibit slower bone therapeutic and reworking. Subsequently, extending the statement interval to evaluate long-term bone reworking and practical outcomes is effective for the practical analysis and future medical translation of engineered EV. In gentle of those factors, we plan to design a extra rigorous experimental protocol and lengthen the statement interval in future research.
To summarize, we developed engineered EV with twin features for stem cell recruitment and osteogenic results each in vitro and in vivo. As the worldwide inhabitants ages, bone-related ailments have more and more grow to be a significant social downside threatening human well being. EVs and engineered EVs maintain nice potential to be new methods for treating bone-related ailments attributable to their superior biocompatibility, organic barrier penetration, excessive loading effectivity, managed launch and improved therapeutic results. Nevertheless, a number of challenges should nonetheless be addressed for medical translation. As an illustration, environment friendly procedures for enriching practical cargo into EV stay a problem. Quite a lot of engineering strategies have been proposed to realize environment friendly loading of endogenous medication in EV [69], however the cargo sorting mechanisms of EV want additional investigation. In addition to, the bone-targeting potential of engineered EV has solely been demonstrated on the cell and animal ranges [70]. Extra medical trials ought to be designed and developed to validate the bone-targeted therapeutic results. Moreover, environment friendly purification and large-scale manufacturing of clinical-grade EV stay one other problem. There may be an pressing want for Good Manufacturing Observe (GMP) pointers for the manufacturing of EV. As well as, 3D cultivation techniques, resembling suspension or organoid cultivation, might enhance the output of engineered EV and assist higher discover the mechanisms of EV in bone microenvironment [71]. Regardless of these challenges, engineered EV maintain nice potential as a therapeutic technique for senescent and different bone-related ailments.
Apt-376b-EV@GelMA enhances senescent vital bone defect restore in vivo. (A) Schematic illustration of the applying of Apt-376b-EV@GelMA for mandibular vital bone defect in aged rats. (B-C) Micro-CT evaluation of bone formation on the defect websites (B) and its quantification of bone quantity/whole quantity (BV/TV) (C) after 8-week operation. (D-G) The bone regeneration analysis of Apt-376b-EV@GelMA after 8 weeks post-implantation by HE (D) and Masson staining (E), and their quantification (F-G). (H-J) Immunofluorescence staining photographs (H) and quantitative evaluation (H-I) of OPN-positive and OCN-positive areas induced by the Apt-376b-EV@GelMA hydrogel in vital bone defects of aged rars. Knowledge represented as imply ± SD (n = 6). The importance of the information was calculated by the one-way ANOVA. (BD, Bone Defect; NB, New Bone. *signifies vital variations in contrast with PBS@GelMA group; # signifies vital variations in contrast with EV@GelMA group; & signifies vital variations in contrast with 376b-EV@GelMA group; p < 0.05. Scale bar, 100 μm. )