The PEPPDL1 is designed to concurrently bind each PD-L1 and Hsc70
The oncogene protein CMTM6 is ubiquitously expressed and competes with the chaperonin protein Hsc70, answerable for lysosome supply, in binding to PD-L1 (Fig. 1A) [32]. This interplay prevents PD-L1 from being degraded by lysosomes, thereby sustaining its excessive mobile abundance [32]. The purpose right here was to beat this impediment and harness the potential of Hsc70, which facilitates cargo supply into lysosomes for degradation by binding with lysosome related membrane protein 2 (LAMP2) [33]. To realize this, PEPPDL1 was ingeniously designed to focus on the PD1 binding area of PD-L1 as a substitute of the Hsc70/CMTM6 binding area (Fig. 1A). As depicted in Fig. 1B, PEPPDL1 was meticulously crafted by combining three practical motifs: a D-type PD-L1 binding motif to work together with its PD-1 binding area [34, 35], a polyethylene glycol linker [28, 36, 37], and an Hsc70 binding motif [38].
The PEPPDL1 is designed to concurrently bind each PD-L1 and Hsc70. A. Schematic diagram of PEPPDL1 peptide design that reactivates lysosomal degradation of PDL1 and blocks PD1. B. Schematic diagram of the synthesis of PEPPDL1. (C) The highest 5 attainable conformations of the PDL1 ligand moiety of PEPPDL1 certain to PDL1. (D) Mimic binding construction and native magnification of the PD1-binding area of PD-L1 in advanced with the PD-L1 ligand. (E) Ramachandran plot of PEPPDL1/PD-L1 advanced. F&G, RMSD (F) and SASA (G) of PEPPDL1/PD-L1 advanced over time within the simulation course of. H. Docking and interactions of the PEPPDL1/PD-L1 advanced with Hsc70. I. Gibbs vitality and interplay space of the highest 5 docked conformations in H. J. Ramachandran plot of the ternary advanced (PEPPDL1/PD-L1/Hsc70 advanced). Ok&L, RMSD (Ok) and SASA (L) of PEPPDL1/PD-L1/Hsc70 advanced over time throughout simulation (Crimson line is the smoothed results of the black line in G&L)
To elucidate the binding mechanism between PEPPDL1 and PD-L1, molecular docking was performed utilizing Discovery Studio. As depicted in Determine S1, the unbound state of PEPPDL1 adopts a random coil construction as a result of proline-induced secondary construction interference. Subsequently, versatile docking was carried out between PEPPDL1 and PD-L1 on the PD-1 binding area, revealing the highest 5 potential docking conformations (Fig. 1C and D and S2). Furthermore, the Ramachandran plot evaluation of the optimum PEPPDL1/PD-L1 conformation as proven in Fig. 1D demonstrated that roughly 99.1% of residues have been positioned throughout the permissible area, with 86.1% falling in probably the most favorable area (blue), 12.2% in extra permissive areas (mild blue), and solely 0.7% in generously permissive areas (white) (Fig. 1E). Its RMSD knowledge (Fig. 1F) indicated that the system reached equilibrium, suggesting a secure and dependable conformation was achieved. Furthermore, after reaching regular state, smoother fluctuations have been noticed within the system peak, reflecting sturdy binding interactions throughout receptor binding. Moreover, SASA values remained persistently secure with out vital deviations throughout general SASA measurements (Fig. 1G). These outcomes collectively assist the reliability of the anticipated PEPPDL1/PD-L1 conformation. Additional evaluation of this conformation reveals that PEPPDL1 (-6.5 kcal/mol) reveals considerably larger affinity in comparison with PD-1 (-4.4 kcal/mol). By using the Van’t Hoff equation formulation, it may be calculated that the theoretical binding fixed of PEPPDL1 is larger than that of the PD-1 (Determine S3). Furthermore, each PEPPDL1 and PD-1 have overlapping binding websites on PD-L1 throughout the similar area, suggesting that PEPPDL1 could function a potent inhibitor for blocking the interplay between PD-1 and PD-L1. As well as, docking simulation within the presence of PEPPDL1, PDL1 and Hsc70 collectively had been carried out to completely perceive the binding mode, displaying the comparatively secure PD-L1/PEPPDL1/ Hsc70 advanced after 50 ns molecular dynamic (MD) time (Determine S4 A&B). Then, the binding of PD-L1 motif was stably certain to PD-L1, and the binding of KEFRQ motifs to Hsc70 additionally introduced a secure pattern (Determine S4C&D). The kinetic binding of anti-PDL1 antibody to PD-L1 and PEPPD-L1 to PD-L1 (Okd of two.44 ± 0.60 µM vs. Okd of 0.64 ± 0.15 µM) was decided by floor plasmonic resonance (SPR) assay (Determine S5A&B), demonstrating superior affinity of PEPPD-L1 to PD-L1 in comparison with anti-PDL1. In the meantime, the kinetic binding of PEPPD-L1 to Hsc70 (with a Kd of 13.45 ± 1.92 µM) additionally demonstrated the precise affinity of PEPPD-L1. This affinity was larger than that of PEPPD-L1 with out the linker (PEG) to Hsc70 (with a Kd of 58.63 ± 12.38 µM), suggesting that the absence of the linker may lower the affinity for the goal protein HSC70 (Determine S5C&D). This is likely to be ascribed to the closeness of the PD-L1 binding sequence to the HSC70 binding sequence, which reduces the binding affinity for the HSC70 motif. And the PEPPDL1, when utilizing the SPR sensor modified with albumin which was offered because the damaging management, confirmed a Okd of 1307.02 ± 46.04 µM (Determine S6). This means that PEPPDL1 has a greater particular choice potential for the goal protein HSC70/PDL1. The outcomes confirmed {that a} excessive stage of PEPPD-L1 may competitively antagonize the binding between Hsc70 and PD-L1. Typically, the aggressive binding property of PD1 was weaker than PD-L1.
The Hsc70 protein was subsequently docked to the anticipated conformation of PEPPDL1/PD-L1 (Fig. 1H). Notably, the KEFRQ motif in PEPPDL1 exhibited a half-turn α-helix conformation, thereby facilitating its interplay with Hsc70 (Determine S8A). Furthermore, the optimum conformation displayed an interface space of 378.3 Å2 and a ∆G worth of -5.2 kcal/mol for the interplay between PEPPDL1/PD-L1 and Hsc70 (Fig. 1H&I). This commentary means that the PEPPDL1/PD-L1 advanced can particularly acknowledge Hsc70 proteins focused for CMA-mediated degradation by way of a KFERQ-like motif (Fig. 1H & S8B). The rationality of the conformation was demonstrated by its Ramachandran plot, which confirmed that 98.4% of residues have been positioned in permissive areas and there have been hardly any residues within the disallowed area (Fig. 1J). Moreover, the curve depicting the system’s RMSD knowledge reached equilibrium at a worth of 0.9 nm through the 5000 ps simulation (Fig. 1Ok), offering additional assist for its rationality. The common SASA values for the conformation have been decided to be 192.5 Å2 (Fig. 1L), with no vital modifications noticed in SASA values over time. Consequently, the docking conformation for PEPPDL1/PD-L1/Hsc70 advanced seems extremely dependable, indicating that PEPPDL1 acts as a bridge to bind each PD-L1 and Hsc70.
The development and characterization of CTAC-PDL1
Subsequent, PEPPDL1 was synthesized by strong section peptide synthesis (SPPS) utilizing Fluorene methoxyl (Fmoc)-protected L- or D- amino acids upon HBTU/HOBT mediated condensation response [31, 39, 40], and their purity and molecular weights have been recognized by liquid chromatography-mass spectrometer (Determine S8). The PEPPDL1 was nanoengineered right into a supramolecular nanosphere (CTAC-PDL1) to confer it with the aptitude of internalization into tumor cells, leveraging the lively uptake of nano proteins by these cells [23, 41]. To realize this goal, a cysteine (Cys) residue was launched on the carboxy terminal of PEPPDL1, adopted by a one-step gentle response to switch the monovalent gold ion (Au(I)) with the terminal sulfhydryl group in Cys. Subsequently, this modification involving sulfydryl and monovalent gold ion (Au(I)) could promote the self-assembly of modified PEPPDL1 into supramolecular nanospheres by way of aurophilic interactions [29, 30, 42], resulting in the formation of CTAC-PDL1 (Fig. 2A). This profitable self-assembled development was additional validated by Fourier Remodel Infrared (FT-IR) spectroscopy (Fig. 2B) and ultraviolet absorption (UV–Vis) spectrum (Fig. 2C). The infrared spectra of PEPPDL1 and CTAC-PDL1 confirmed similar absorption at 1680 cm− 1 (C = O) and 3300 cm− 1 (N-H), which was brought on by the amide bonds of PEPPDL1 in CTAC-PDL1 (Fig. 2B). Additional, the infrared spectra of CTAC-PDL1 revealed an absorption at 2950 cm− 1 (Au1+-SR), suggesting that the thiol peptide and Au ions are copolymerizing. This outcome was confirmed once more by the attribute absorption peaks of peptides in UV–Vis spectrum at 280 nm (Fig. 2C), confirming the fabrication technique of CTAC-PDL1 which is in step with our expectations.
The development and characterization of CTAC-PDL1. (A) Schematic displaying the self-assembly of CTAC-PDL1. (B) Fourier remodel infrared (FT-IR) of PEPPDL1 and CTAC-PDL1. (C) Characterization of synthesized PEPPDL1 and CTAC-PDL1 by UV-Vis spectrum. (D) TEM pictures of CTAC-PDL1. (E) Elemental evaluation picture of S, O, N, Au overlay with one consultant particle of CTAC-PDL1 taken by HRTEM. (F) EDS quantitative component evaluation of CTAC-PDL1. G&H. Hydrodynamic diameter (G) and ZETA potential (H) of CTAC-PDL1 assembled nanocluster measured in PBS buffer at pH 7.4. I.PEPPDL1 launch curve of CTAC-PDL1 in 10 mM or 10 µM GSH PBS buffer, measured by HPLC. Information was introduced because the imply ± SD. J. The soundness of CTAC-PDL1 in 20% serum persevering with 24 h
Furthermore, Transmission electron microscope (TEM) pictures present that CTAC-PDL1 is comparatively uniform in dimension and reveals favorable monodisperse traits (Fig. 2D). As well as, we carried out elemental evaluation and diffraction evaluation on CTAC-PDL1 by way of a high-resolution transmission microscope. Power Dispersive X-Ray Spectroscopy (EDS) evaluation signifies that the weather of CTAC-PDL1 are in step with these of its reactants (Fig. 2E). The superposition pictures of vibrant fields and varied components below the identical view present that the gold (Au), nitrogen (N), oxygen (O), and sulfur (S) components in CTAC-PDL1 are uniformly distributed, which signifies the homogeneity of peptides and gold within the nanoparticles (Fig. 2F). The dynamic mild scattering (DLS) evaluation confirmed that CTAC-PDL1 had a median hydrodynamic diameter of 20.9 nm in a slender peak distribution with the acceptable polydispersity index (PDI) worth (0.13 ± 0.02), additional illustrating that CTAC-PDL1 has cheap uniformity in dimension (Fig. 2G). Furthermore, the ζ potential of CTAC-PDL1 was 8.5 mV (Fig. 2H), consistent with the electro positivity of PEPPDL1. The required designed operate of CTAC-PDL1 is to controllably launch cargos contained in the focused cell. To confirm the stimuli responsive cargo launch triggered by the differential focus of GSH, analytical HPLC was used to watch the discharge kinetics of CTAC-PDL1 (Fig. 2I). In PBS buffer containing 10 µM GSH at pH 7.4, CTAC-PDL1 typically preserve their integrality with < 20% cargo launch after 12 h incubation. In sharp distinction, including GSH to 10 mM resulted within the disintegration of CTAC-PDL1 and subsequent ~ 80% cumulative launch inside one other 6 h, indicating a GSH-concentration-dependent cargo launch. Lastly, the hydrodynamic diameter throughout 24 h is almost constant 20–30 nm, which carried out the relative stability of CTAC-PDL1 (Fig. 2J).
Pores and skin cutaneous melanoma (SKCM) was recognized as probably the most appropriate indication for CTAC-PDL1
PD-L-dependent most cancers immune evasion usually have a attribute of a lot of dysfunctional tumor-infiltrating CD8 + T cells and highly effective regulatory T (Treg) cells. To hunt out the very best beneficiaries of PD-L1 degradation and probably the most applicable mode for CTAC-PDL1 remedy, the connection between the expression of neoplastic PD-L1 and Tumor Infiltrating Lymphocytes (TILs) amongst 30 frequent tumors have been analyzed by The Most cancers Genome Atlas (TCGA). First, as proven in Fig. 3A, densities of each tumor-infiltrating CD8 + T cells and Treg cells had the strongest PD-L1-related correlation in pores and skin cutaneous melanoma (SKCM), suggesting that SKCM sufferers is likely to be potential beneficiaries of PD-L1 degradation. Moreover, Treg cells and Treg-related immunosuppressive components, IDO and IL-10 have been positively associated to PD-L1 expression (Fig. 3B). Furthermore, in sharp distinction to the PD-L1-related correlation of tumor-infiltrating CD8 + T cells in SKCM, immunostimulatory CD276 and PVR have been negatively associated to PD-L1 expression (Fig. 3C). These outcomes illustrated that PD-L1 may cause the operate activation of Treg cells in addition to the dysfunction of CD8 + T cells and the following immunosuppression. On this case, the intrinsic regulatory protein HIP1R that targets PD-L1 to lysosomal degradation [16] was negatively associated to PD-L1 expression, demonstrating that the inartificial regulating system of PD-L1 was dysfunctional in immunosuppressive SKCM (Fig. 3D). Then, searching for the suitable various for PD-L1 to degradation, we analyzed the expression of 19 protein involving in protein degradation in SKCM (Fig. 3E) in relationship with the PD-L1 expression. The expression of Hsc70 in SKCM exhibited a strong constructive correlation with PD-L1, whereas the mRNA ranges of Hsc70 have been larger in tumor tissues in comparison with adjoining regular tissues (Fig. 3F). Importantly, this constructive correlation is probably going attributed to the inhibition of Hsc70-mediated degradation of PD-L1 by CMTM6 and subsequent upregulation of Hsc70 induced by PD-L1 interplay with tumor cells. What’s extra, consistent with the Hsc70 expression, each LAMP2 (Lysosomal Related Membrane Protein 2) and LAMP5 (Lysosomal Related Membrane Protein 5) that take part within the Hsc70-involved lysosomal degradation have been additionally positively associated to PD-L1 expression in SKCM (Fig. 3G). As a result of CMTM6 facilitated PD-L1 again to plasma membrane and competitively certain PD-L1 with Hsc70 [17], we assessed the correlation gene expression of CMTM6, CD274 and HSPA8 in GEPIA database. The outcomes confirmed that CMTM6 was positively correlated with the expression of CD274 and HSPA8 in SKCM (Fig. 3H).
Pores and skin cutaneous melanoma (SKCM) was recognized as probably the most appropriate indication for CTAC-PDL1. A. Heatmap of spearman correlation between expression of PD-L1 and TILs throughout human cancers. B. The correlation between expression of PD-L1 and immunoinhibitor together with Treg abundance, IDO (Indoleamine 2,3-dioxygenase) and IL-10 expression respectively in SKCM. (C) The correlation between expression of PD-L1 and immunostimulator together with lively CD8 abundance, CD276 and PVR (Poliovirus receptor) expression in SKCM. (D) The correlation between expression of PD-L1 and HIPIR in SKCM; (E) Bubble diagram and Heatmap of correlation between expression of PD-L1 and a number of molecular chaperones in SKCM. (F) The expression amount of Hsc70 between tumor and regular tissue in SKCM. (G) The correlation between expression of PD-L1 and Hsc70, LAMP2 and LAMP5 in SKCM. (H) Correlation evaluation between CMTM6 and CD274, Hsc70 in GEPIA database in SKCM. (I) Schematic illustration for restoration of PD-L1 degradation to manage anti-tumor exercise
Collectively, the bioinformatics evaluation in line with the TCGA knowledge set and GEPIA database verified that the intrinsic Hsc70/LAMP-related PD-L1 lysosomal degradation pathway was suppressed in PD-L1-overexpressed tumor with immune escape particularly in SKAM (n = 472). Therefore, using CTAC-PDL1 to impede CMTM6-mediated elevated mobile abundance of PD-L1 and regulate the extent of PD-L1 by way of Hsc70/LAMP-derived lysosomal degradation pathway may probably provide a tumor-specific method in SKAM (Fig. 3I) for focusing on PD-L-dependent most cancers immune evasion.
CTAC-PDL1 degraded PD-L1 through Hsc70/LAMP2-derived lysosomal degradation
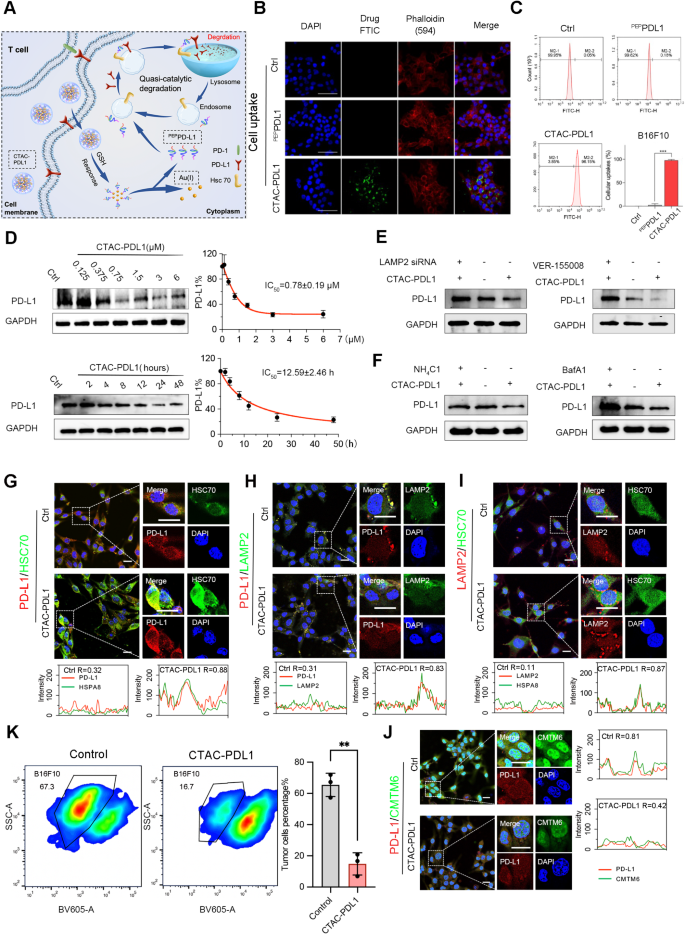
The CTAC-PDL1 design allows environment friendly intracellular supply and managed launch of PEPPDL1 in response to elevated ranges of intracellular GSH. Subsequently, the launched PEPPDL1 interacts with intracellular PD-L1 and Hsc70, facilitating PD-L1 lysosomal degradation by way of the Hsc70-mediated CMA pathway (Fig. 4A). To guage the internalization potential of CTAC-PDL1 into melanoma cells, fluorescein isothiocyanate (FITC) was conjugated to the N-terminal of PEPPDL1, and this FITC-labeled PEPPDL1 was additionally used to assemble fluorescent CTAC-PDL1. Subsequently, B16F10 melanoma cells have been incubated with FITC-labeled PEPPDL1 and CTAC-PDL1 for 0, 0.5 h, 1 h, 2 h, 4 h and 6 h. As indicated by the outcomes of uptake with time (Fig. 4B and Determine S9), vital intracellular uptake was noticed after 1 h after CTAC-PDL1FITC remedy, in comparison with PEPPDL1FITC. This discovering of 6 h cell uptake was additional confirmed by quantitative stream cytometry evaluation proven in Fig. 4C. Previous to initiating CTAC-PDL1-mediated Hsc70-dependent PD-L1 lysosomal degradation, we had carried out co-immunoprecipitation (CO-IP) to verify the interplay of CTAC-PDL1 to PD-L1 and Hsc70, as illustrated in Determine S10, which was in step with the outcomes of molecular dynamics. Fluorescence colocalization between FITCCTAC-PDL1 and PD-L1 confirmed CTAC-PDL1 exhibited the flexibility to bind with PD-L1 (Determine S11), thereby it may acknowledge by the initiator protein Hsc70 in CMA-mediated lysosomal degradation. Subsequently, CTAC-PDL1 may primarily binds to PD-L1 and Hsc70 within the cytoplasm and intracellular vesicles, similar to lysosomes, early endosomes, and late endosomes [43, 44]. These outcomes exhibit that the technique employed to rework PEPPDL1 right into a supramolecular nanosphere enhances its tumor cell internalization functionality.
CTAC-PDL1 degraded PD-L1 through Hsc70/LAMP2-derived lysosomal degradation. A. Schematic diagram of lysosome-dependent degradation of PD-L1 in response to CTAC-PDL1 remedy in tumor cells. B&C. Mobile uptake of FITC-labeled PEPPDL1 and CTAC-PDL1 into B16F10 cells after 6 h incubation measured by Immunofluorescence with Laser Scanning Confocal Microscopy pictures (B) and stream cytometry (C) (Scale bar: 100 μm). D. After incubation with completely different focus of CTAC-PDL1 for twenty-four h or completely different time of CTAC-PDL1 (0.75 µM), the expression of PD-L1 was measured by westen blot in B16F10 melanoma cells. E.The PD-L1 stage responded to CTAC-PDL1 (50 µg/mL) with or with out LAMP2-siRNA and VER-155,008 (HSPA8 inhibitor, 2.6 µM) by westen blot. F. The PD-L1 stage responded to CTAC-PDL1 (50 µg/mL) with NH4Cl (250 mg/mL) or BafA1 (1 µM). G-J. immunofluorescence confirmed the colocalization between PD-L1 and HSPA8 (G), PD-L1 and LAMP2 (H), LAMP2 and HSPA8 (I) or CMTM6 and PD-L1 (J) after handled with CTAC-PDL1 (50 µg/mL) for 12 h. Ok. Movement cytometry evaluation of percentages of tumor cells after CD8 + T cell co-cultured with B16F10 with or with out CTAC-PDL1 (50 µg/mL) remedy. (**, p < 0.01)
To research the biofunction of CTAC-PDL1, the PD-L1 abundance in B16F10 cells was semi-quantified by Western Blot evaluation. As demonstrated in Fig. 4D, CTAC-PDL1 carried out a dose-dependent discount in PD-L1 after a 24 h incubation with an IC50 worth for CTAC-PDL1 focus estimated at 0.78 ± 0.19 µM. In the meantime, CTAC-PDL1 additionally had proven good efficiency of PD-L1 inhibition in each shorter and longer intervals (IC50 = 12.59 ± 2.46 h) (Fig. 4D and Determine S12). Subsequently, CTAC-PDL1 may cut back PD-L1 expression in a time-dependent and dose-dependent method, indicating that CTAC-PDL1 performed a sustainable position in selling CMA-mediated lysosomal degradation of PD-L1. To research the biofunction of CTAC-PDL1 to PD-L1, we employed LAMP2 small interfering RNA (siRNA), Hsc70 inhibitor and lysosome inhibitor to entry PD-L1 expression. LAMP2 knockdown effectivity was carried out by Western Blot and Q-RTPCR (Determine S13). The lysosomal-dependent degradation of PD-L1 was confirmed by observing the practically full reversal of CTAC-PDL1-induced PD-L1 degradation in B16F10 cells when co-incubated with LAMP2 siRNA, Hsc70 inhibitor (VER-155008), lysosomal inhibitors (NH4Cl or bafilomycin A1, BafA1) utilizing Western Blot evaluation (Fig. 4E&F). It was confirmed that Hsc70 inhibitor and sturdy lysosomal inhibitor BafA1 carried out higher inhibitory impact of PD-L1. Subsequent, LAMP2 addback experiment was carried out by LAMP2 agonist (QX77) in Figures S14A&B, which confirmed elevated LAMP2 and decreased PD-L1 expression after QX77 administration particularly for LAMP2 knocked down. QX77 additionally performed a constructive position in PD-L1 degradation which has similarities to CTAC-PDL1 (Determine S14C). General, these findings persistently assist the position of CTAC-PDL1 in lowering PD-L1 ranges by way of Hsc70-mediated lysosomal degradation.
As a result of CTAC-PDL1/PD-L1 advanced acknowledge Hsc70 proteins by way of a KFERQ-like motif to mediate CMA-mediated degradation (Fig. 4A), the incremental fluorescence colocalization between PD-L1 and Hsc70 in CTAC-PDL1-treated B16F10 cell. In the meantime, CTAC-PDL1 administration additionally confirmed exceptional enhance within the fluorescence colocalization amongst PD-L1, LAMP2 and Hsc70 in comparison with management group (Fig. 4G – I), indicating the degradation of PD-L1 in CMA-mediated lysosomal degradation. Moreover, so as to observe the connection between the CTAC-PDL1 and the lysosome significantly, we carried out FITC labeled CTAC-PDL1 (FITCCTAC-PDL1) with Lysotracker after co-incubation with B16F10 in 6 h. The outcomes of colocalization additionally indicated that CTAC-PDL1 may obtain lysosomal-mediated protein degradation, which may very well be counteracted by the Hsc70 inhibitor (Determine S15). As depicted in Fig. 4J, decreased fluorescence colocalization between PD-L1 and CMTM6 after CTAC-PDL1 remedy, implying blocked CMTM6-mediated PD-L1 re-back to membrane by CTAC-PDL1. In abstract, CTAC-PDL1 is able to successfully lowering the protein ranges of PDL1 within the cytoplasm, thereby reducing the switch of PD-L1 to the cell membrane.
To research the antitumor impact of CTAC-PDL1 by CD8 + T cell, B16F10 tumor cell co-cultured with CD8 + T cell with or with out CTAC-PDL1 remedy to detect percentages of tumor cells by stream cytometry. As we anticipated, CTAC-PDL1 remedy (50 µg/mL) decreased variety of B16F10 in comparison with management group (p < 0.01), demonstrating CTAC-PDL1 may facilitate CD8 + T cell-mediated cytotoxicity to focus on tumor cells (Fig. 4Ok).
CTAC-PDL1 successfully induced degradation of PD-L1 in vivo and revitalized the acquired immune response of T cells
To research the efficacy of CTAC-PDL1 in treating melanoma in vivo, C57BL/6 mice with subcutaneous B16F10 melanoma tumors on their flanks have been initially handled subcutaneously (Fig. 5A). As soon as the tumor quantity reached 50–100 mm3, the mice have been randomly divided into three teams to obtain intravenous remedies each different day for eight days. The remedy teams included a PBS management, a murine PD-1 monoclonal antibody (Anti-PD1, 2 mg/kg), and CTAC-PDL1 (2 mg/kg). In contrast with little distinction between murine PD-1 monoclonal antibody (Anti-PD1) remedy to PBS management, The immunohistochemistry rating considerably decreased after CTAC-PDL1 remedy (Fig. 5B&C). In the meantime, the IHC rating of PD-L1 in Fig. 5C indicated that, primarily based on the statistical evaluation of a number of samples, there have been no variations between the anti-PD-1 antibody handled group and the management group. The above analysis outcomes pattern was in step with the PD-L1 protein expression proofed by Western blotting, which was superior to murine PD-1 monoclonal antibody remedy (Determine S16).
CTAC-PDL1 successfully induced degradation of PD-L1 in vivo and revitalized the acquired immune response of T cells. A. Diagrammatic sketch of the B16F10 orthotopic melanoma mannequin in C57BL/6 mice and the dosing routine. (B16F10, 4*105 cells/mice). B&C. Immunohistochemical pictures (B) and IHC rating (C) of PD-L1 in mice of B16F10 melanoma after indicated remedy (Scale bar: 50 μm). D&E. T-SNE plots displaying mobile landscapes primarily based on detailed cell typing in most cancers cells each markers in cell varieties (D) and proportion of cell varieties (E). F. T-SNE plots displaying the proportion of CD4 + and CD8 + cells in most cancers cells. G. UMAP plots displaying the expression of chosen marker genes in CD8 + T cells. H. multichannel stream cytometry evaluation of Treg in SKAM after completely different remedies. I. multichannel stream cytometry evaluation of CTL cells in in SKAM after completely different remedies. J. The proportion of cell sort after multichannel stream cytometry evaluation. (*, p < 0.05; **, p < 0.01; ***, p < 0.001, ns imply no vital distinction)
The exfoliated melanoma tumors have been subjected to single-cell RNA sequencing subsequent, so as to examine the influence of CTAC-PDL1-derived PD-L1 degradation on the tumor immune microenvironment. In all three teams, eight distinct cell varieties have been recognized primarily based on their marker genes (Fig. 5D), and the single-cell expression atlas (n = 3/group) is introduced (Fig. 5E). The evaluation of T lymphocytes signifies a rise in CD8-positive T lymphocytes (CD8 + T) and an elevated CD4+/CD8 + ratio following remedy with CTAC-PDL1, which demonstrates superiority over murine PD-1 monoclonal antibody remedy (Fig. 5F). CD8 + T cells play a vital position in anti-tumor immunity by eliminating most cancers cells and inducing apoptosis by way of granzyme secretion. Additional evaluation reveals vital upregulation of interferon γ (IFN γ), granzyme B (GamB), and Perforin 1 (Pfn1) in CD8 + T cells after CTAC-PDL1 remedy, suggesting a reversal of CTL exhaustion (Fig. 5G). The outcomes have been additional supported by multichannel stream cytometry, revealing a lower in intratumoral Treg cells following CTAC-PDL1 remedy (Fig. 5H). In the meantime, the degrees of CD8 + cytotoxic T cells and the chances of (GzmB+) and (IFN γ+) CD8 + cells considerably elevated in tumors handled with CTAC-PDL1 (Fig. 5H-J). These collective findings exhibit the efficient induction of lysosomal degradation of PD-L1 by CTAC-PDL1, thereby rejuvenating the acquired immune response of T cells in melanoma.
CTAC-PDL1 exhibited vital anti-tumor efficacy
The one-cell expression atlas in tumor cell clusters offered proof for the anti-tumor exercise of CTAC-PDL1, as demonstrated by the noticed lower in mobile proliferation and enhance in cell demise (Fig. 6A-D). Intimately, the proportion of Ki67/PCNA constructive cells was considerably decreased in tumors handled with CTAC-PDL1, indicating suppressed mobile proliferation (Fig. 6A). Conversely, there was a marked enhance in Ripk1/3/MIKI and Cspa3/Cspa8 constructive cells, demonstrating enhanced programmed cell demise particularly by way of necroptosis and pyroptosis upon remedy with CTAC-PDL1 (Fig. 6B&C). Moreover, the marker of ferroptosis (Chac1/Slc7a11) additionally exhibited a rise to 26.8% for each murine PD-1 monoclonal antibody and CTAC-PDL1 in comparison with the management group’s worth of 21.4% (Fig. 6D). The H&E staining revealed that CTAC-PDL1 exerted a potent and memorable suppression on tumorous histological options (Fig. 6E). Moreover, TUNEL staining of tumor tissue sections demonstrated that CTAC-PDL1 remedy considerably enhanced tumor apoptosis in comparison with murine PD-1 monoclonal antibody and PBS remedies (Fig. 6F). Primarily based on the analysis of tumor picture (Fig. 6G), tumor weights measured on the remaining day (Determine S17), in addition to each day monitoring of tumor quantity (Fig. 6H), it was noticed that the CTAC-PDL1 group exhibited a extra pronounced impact in suppressing tumors than the murine PD-1 monoclonal antibody group and Management group by the tip of remedy, with a twofold enhance in TGI in comparison with murine PD-1 monoclonal antibody remedy (Fig. 6H).
CTAC-PDL1 exhibited vital anti-tumor efficacy towards B16F10-derived mice mannequin of malignant melanoma. A-D. UMAP plots displaying the expression of chosen marker genes in most cancers cells of proliferation (A), necroptosis (B), apoptosis (C) and ferroptosis (D). E. Consultant H&E staining photographs tumor sections. F. TUNEL staining pictures and quantitative evaluation of tumor tissue sections after indicated remedy. G. Consultant photographs of the tumors remoted from mice on the finish of the experiment. H. Tumor development curves of Management, murine PD-1 monoclonal antibody and CTAC-PDL1 in mice subcutaneously inoculated with melanoma. Information are introduced as Imply ± SEM. (n = 5/group). I-L. The Immunohistochemical pictures and IHC Rating of CD80 (I), Perforin-1 (J), Granzyme A (Ok) and Granzyme B (L) in tumor sections with the indicated completely different remedies in mice of B16F10 melanoma. (Scale bar: 50 μm. *, p < 0.05; **, p < 0.01; ***, p < 0.001)
Furthermore, immunohistochemical (IHC) evaluation of T cell secretory proteins, together with CD80, Perforin-1, Granzyme A and Granzyme B, additional helps the anti-tumor motion ensuing from the acquired immune response of T cells (Fig. 6I-L). Particularly, our outcomes exhibit that CD80 expression is upregulated after CTAC-PDL1 remedy (Fig. 6I), which aligns with earlier studies. These findings collectively exhibit that CTAC-PDL1 successfully degrades tumor PD-L1 and reactivates T-cell immunity. It’s well-known that cytotoxic T lymphocytes (CTLs) are immune cells able to recognizing and eliminating most cancers cells by way of the discharge of specialised cytolytic proteins similar to perforin and granzyme [45]. As depicted in Fig. 6I-L, CTAC-PDL1 considerably enhances intratumoral ranges of Perforin-1, Granzyme A and Granzyme B in comparison with the Management group. In distinction, murine PD-1 monoclonal antibody serves as a constructive management however solely marginally will increase these indicators.
Importantly, so as to certify the therapeutic results of CTAC-PDL1 amongst distinction expression of PD-L1 in vivo, we established PD-L1 knockout MC38 cell line by CRISPR-Cas9 (Determine S18A). And MC38 (WT) and PD-L1 knocked out MC38 cells (KO) have been subcutaneous injected to C57BL/6 mice to kind MC38 colon adenocarcinoma (COAD) subcutaneous tumor. CTAC-PDL1 was administered to mice and monitored physique weight on a regular basis (Determine S18B). The PD-L1 knockout COAD tumors confirmed considerably suppressed tumor development in comparison with the MC38 tumors expressing PD-L1 (Determine S18C). In the meantime, the wild-type COAD tumors demonstrated elevated anti-tumor immune response after CTAC-PDL1 remedy fairly than PD-L1 knockout COAD (Determine S18 C-E). The findings illustrated that the CTAC-PDL1could improve anti-tumor immune response focusing on PD-L1.
For biosafety concerns to validate the pharmacokinetics of CTAC-PDL1, B16F10 cells have been injected to mice through subcutaneous injection (5 × 105 cells/mice) and FITCCTAC-PDL1 (2 mg/kg) was administrated to facilitate in vivo quantification by ICP-MS. CTAC-PDL1 possessed a passable half-life in vivo. In the meantime, CTAC-PDL1 is metabolically suitable with the most important organ (coronary heart, liver, spleen, kidneys, lungs) and tumors (Determine S19). IVIS spectrum carried out to entry organ distribution of medicine by FITCCTAC-PDL1 in wholesome mice inside 24 h. The outcomes indicated that CTAC-PDL1 amassed primarily within the liver and kidney. And the fluorescence depth decreased over time, suggesting that the polypeptide may need the passable scientific security (Determine S20). Lastly, we now have demonstrated the toxicity of the supramolecular peptide CTAC-PDL1 in vivo. Wholesome C57BL/6 mice have been divided into three teams and given CTAC-PDL1 or murine PD-1 monoclonal antibody intraperitoneally, as soon as each different day, 5 remedy cycles (CTAC-PDL1, 5 mg/kg). Physique weight monitoring didn’t carry out evident notable weight reduction within the CTAC-PDL1 group and murine PD-1 monoclonal antibody group (Determine S21). Apart from, the administration of CTAC-PDL1 for 10 days didn’t end in any vital physique weight reduction or modifications within the H&E staining of organs (Determine S22), highlighting the impeccable security profile of CTAC-PDL1. Routine blood and biochemistry exams have been carried out to certify the biosafety of hemopoietic system. CTAC-PDL1 didn’t have an effect on purple blood cells (RBC), white blood cells (WBC), platelets (PLT), hemoglobin (HGB), granulocytes (GRAN), monocytes (Mon) or lymphocytes (Lymph) in mice compared with controls (Determine S23). Glutamic-pyruvic transaminase (ALT), Glutamic-oxalacetic transaminase (AST), blood urea nitrogen (BUN) and creatinine (CRE) have been assessed to watch hepatotoxicity and nephrotoxicity (Determine S24). In mixture, these findings unequivocally exhibit that CTAC-PDL1 reveals exceptional anti-tumor efficacy in vivo whereas sustaining an exceptionally favorable biosafety profile.