To analyze intermolecular interactions between peptides and polyphenols, six homopolypeptides containing completely different aspect chains (i.e., Arg6, His6, Lys6, Asp6, Professional6, and Thr6) have been chosen as mannequin peptides, whereas TA was chosen because the mannequin polyphenol (Scheme 1a). Notably, the amino group within the peptide’s amino acid is definitely protonated and positively charged, whereas the carboxyl group is definitely deprotonated and negatively charged, relying on the isoelectric level of the amino acid and answer pH [29]. Equally, the TA phenolic hydroxyl group can also be simply deprotonated and negatively charged below acidic circumstances [30], which offers preconditions for electrostatic interactions between polyphenols and polypeptides. As well as, the chosen peptides can type different noncovalent interactions, comparable to hydrogen-bonding, or hydrophobic interactions with TA. Subsequently, polyphenol–protein coatings have been obtained by dip-coating and LbL meeting on silicon substrates, and the meeting mechanism was investigated (Scheme 1b).
LbL meeting
Determine 1a reveals the thickness progress of hexapeptides (hexameric amino acids) assembled with positively and negatively charged, uncharged, and hydrophobic TA layers. First, the thicknesses of the layered negatively charged peptide (Asp6), uncharged peptide (Professional6), and hydrophobic peptide (Tyr6) assembled with TA negligibly elevated, whereas the thicknesses of the layered coatings of positively charged peptides Arg6, His6, and Lys6 assembled with TA all considerably elevated, and the thickness of 15 bilayers was related, roughly 40 nm, which signifies that the TA–peptide meeting was dominated by electrostatic interactions, whereas neither hydrogen-bonding nor hydrophobic interactions alone maintained the LbL meeting of TA with peptides. Determine 1b reveals the frequency (f) plotted as a operate of the dissipation (D) throughout the meeting of TA with the positively charged peptide (His6) at pH 7, the place f is inversely proportional to the sensor-coupling high quality, and D is a semiquantitative parameter to specific the “softness” of movies [31]. Throughout the LbL meeting of the TA and positively charged peptide, Δf monotonically decreased, indicating that the TA and peptide have been each deposited on the substrate. Notably, Δf was considerably increased for the TA layer than the peptide, indicating that throughout the LbL meeting, extra TA was deposited than the peptide. Moreover, ΔD monotonically elevated throughout each the TA and peptide assemblies, indicating that the coating grew to become softer and swollen with rising variety of assembled layers. For each the TA and His6 layers, ΔD was low and related, indicating that the TA and His6 layers have been structurally related and that the coatings exhibited comparatively inflexible mechanical properties. As well as, throughout the assemblies of TA with Arg6 (Determine S1a) and Lys6 (Determine S1b), Δf and ΔD confirmed patterns according to that throughout the meeting of TA with His6.
Determine 1c reveals the FTIR spectra for coatings of TA assembled with the positively charged peptide (His6) at pH 7. For TA, the broad peak at 3350 cm− 1 corresponds to the stretching vibration of ph-OH. The height at 1701 cm− 1 corresponds to C = O stretching, and peaks at 1605, 1533, and 1443 cm− 1 correspond to skeletal vibrations of the benzene ring. For the His6 peptide, reasonably intense peaks at 3137 and 3031 cm− 1 are each antisymmetric stretching vibrations of N–H within the amide. The height at 1657 cm− 1 corresponds to the stretching vibration of the amide I band (C = O). The height at 1543 cm− 1 corresponds to the bending vibration of the amide II band (N–H). The height at 1430 cm− 1 corresponds to the absorption of the amide III band (C–N). For TA–His6, the height at 3152 cm− 1 corresponds to the stretching vibration of the amide N–H of His6. The height at 1710 cm− 1 corresponds to the stretching vibration of the ester carbonyl C = O of TA. The height at 1674 cm− 1 corresponds to the stretching vibration of the amide I band of His6 (Fig. 1d). Peaks at 1605, 1503, 1444 cm− 1 correspond to skeletal vibrations of the benzene ring in TA, the place the height at 1503 cm− 1 signifies that some phenolic hydroxyl teams in TA have been oxidized to quinone constructions, and the height at 1444 cm− 1 corresponds to the absorption of the amide III band (C–N) of His6. These outcomes recommend that TA was assembled with His6. Related FTIR spectra have been obtained for the assemblies of TA with Arg6 (Determine S2a) and Lys6 (Determine S2b), indicating that TA was assembled with positively charged peptides.
Because of the excessive oxidative susceptibility of TA, particularly when pH > 7, the phenolic hydroxyl group can type reversible covalent Schiff-base interactions with amines after oxidation to quinone (C = N). [32] His6 was characterised utilizing XPS earlier than (Fig. 1e) and after (Fig. 1f) LbL meeting with TA, and the N 1 s peak was deconvoluted within the His6 spectrum however not within the TA spectrum. The C = N content material was negligibly completely different earlier than and after the LbL meeting, indicating that the coating contained virtually no Schiff-base covalent bonds. Related XPS spectra have been obtained for the assemblies of TA with Arg6 (Determine S3a and b) and Lys6 (Determine S3c and d), and the N 1 s compositions quantitatively analyzed earlier than and after the meeting are listed in Desk S3. The absence of Schiff base within the LbL coating is partly as a result of slower oxidation of TA below impartial circumstances and the formation of much less quinone, and partly as a result of brief meeting time will not be adequate for the formation of Schiff base covalent bonds. As well as, the coating compositions of TA assembled with completely different positively charged peptides have been analyzed utilizing XPS (Desk S4). For Arg6, His6, and Lys6, the proportion of certain TA decreased. This can be as a result of decreases within the variety of protonatable N atoms and the density of optimistic fees on the peptide floor, which weakened electrostatic interactions and, thus, decreased the quantity of certain TA.
As a result of properties, comparable to morphology and mechanical properties, have an effect on organic features of peptide coatings below physiological circumstances, LP–AFM assessments have been performed by immersing peptide coatings in buffer options. LP–AFM allows the analysis of the construction and properties of biologically energetic coatings below physiological circumstances whereas decreasing the quantity of injury to the product [33]. Determine 1g reveals the floor morphology of the (TA–His6)15 coating within the liquid setting. The coating consists of aggregated particles with a discontinuous tough floor construction, and the typical peak of the coating is roughly 40 nm, which is according to the optical thickness measured utilizing ellipsometric polarization spectroscopy. The Younger’s modulus of the (TA–His6)15 coating was additional evaluated. As proven in Fig. 1h, the mechanical properties of the coating have been comparatively uniformly distributed, and a Younger’s modulus of roughly 12.6 ± 3.6 MPa confirmed a Gaussian distribution. Coatings (TA–Arg6)15 and (TA–Lys6)15 additionally exhibited morphologies and Younger’s modulus distributions much like these of (TA–His6)15 (Figs. S4 and S5, respectively). Younger’s modulus was obtained by becoming the LP–AFM pressure curve to the Johnson–Kendall–Roberts (JKR) mannequin, which is a contact mechanics mannequin that considers adhesive interactions within the contact zone [34]. Typical evaluated pressure curves coincided effectively with the JKR mannequin curves, indicating the reliability of the Younger’s modulus calculations (Determine S6). The floor adhesion work of the peptide coatings was then solved utilizing the JKR theoretical equation, which signifies the coating adhesions [35], and the adhesion works of the (TA–Arg6)15, (TA–His6)15, and (TA–Lys6)15 coatings have been 2.7, 4.1, and three.6 µN·m− 1, respectively (Determine S7). As well as, the floor morphology, Younger’s modulus, and adhesion work of the peptide coatings have been evaluated in ambient air (Determine S8 and S9). Though the thicknesses of the peptide coatings obtained within the ambient air and liquid have been negligibly completely different, the Younger’s modulus and adhesion work of the peptide coatings obtained in ambient air have been increased than these of the peptide coatings obtained within the liquid, which is perhaps due to the distinction within the movie hydration in numerous testing environments [26].
(a) Buildup curve for thickness of (TA/hexapeptide)n coating. (b) Actual-time shifts of Δf and ΔD throughout fabrication of (TA/His6)6 coatings. (c) FTIR spectra for chemical constructions of TA/His6, TA, and His6. (d) Detailed FTIR spectrum similar to space in dashed field in (c) for TA/His6. Detailed N 1 s XPS spectra of (e) His6 powder and (f) (TA–His6)15 coating on silicon wafers. (g) LP–AFM picture of (TA–His6)15 coating. (h) Picture and frequency distribution of Younger’s modulus for (TA–His6)15 coating obtained in liquid
Influencing components of LbL meeting
The consequences of the polyphenol and polypeptide species on the LbL meeting have been investigated. Polyphenols having completely different molecular weights (TA, PC, EGCG, Cat, and GA, for which constructions are displayed in Determine S10) have been assembled with peptides. As proven in Fig. 2a, not one of the polyphenols might be assembled with negatively charged (Asp6), uncharged (Professional6), and hydrophobic (Tyr6) peptides. TA, PC, EGCG, which had comparatively excessive molecular weights, might be assembled with positively charged peptides (Arg6, His6, and Lys6), whereas Cat may solely be assembled with Arg6, and GA, which had the bottom molecular weight, couldn’t assemble with positively charged peptides, which signifies that the meeting of polyphenols and peptides primarily is determined by electrostatic interactions and that the molecular weight impacts the power of polyphenols to assemble with peptides. As well as, Arg has barely extra protonatable N atoms than His and Lys and, thus, can type stronger electrostatic interactions with polyphenols.
The consequences of the pH, salt focus, and peptide chain size and sort on the thickness of TA–peptide coatings have been investigated throughout LbL meeting. For the impact of the pH on the thickness of TA–peptide coatings throughout LbL meeting, as proven in Fig. 2b, the meeting of TA with the positively charged peptide (His6) reveals an apparent pH dependence, which is intently associated to the pKa of TA, isoelectric level (pl), and variety of charged amino acids within the peptide. The TA presents two pKa values, pKa1 = 3.3 and pKa2 = 8.7, and the pl of His is 7.6 [36,37,38]. With rising pH, the thickness of the coating wherein TA was assembled with His6 first elevated after which decreased. When the answer is just too acidic (pH < 3), the TA dissociation is inhibited, and the TA cost is diminished, which weakens the electrostatic interplay between TA and His6, leading to virtually no meeting progress of the coating. When the answer pH is above the pl of His6 (pH > 7.6), His6 N atoms protonate, and the His6 cost decreases, which weakens the electrostatic interplay between TA and His6 and slows the expansion of the coating. As well as, for positively charged peptides Arg6 (pl = 10.8, Determine S11a) [39] and Lys6 (pl = 9.7, Determine S11b) [40], the pH dependence of the assemblies with TA confirmed patterns according to the pH dependence of the meeting of His6 with TA, indicating the dominance of electrostatic interactions within the LbL meeting of TA with peptides. The impact of the salt focus on the coating thickness was investigated throughout the meeting of TA with positively charged peptides, as proven in Fig. 2c and Determine S12. LbL meeting negligibly trusted the salt focus, which can be as a result of the molecular weights of the TA and peptides are too low for the salt to successfully defend fees carried by these small molecules, thus rendering LbL meeting insensitive to the salt focus.
The impact of the peptide chain size on the expansion of the assembled coating was investigated by various the variety of amino acids within the peptide. His3, His6, His9, and His12 have been designed and synthesized utilizing the His unit. As proven in Fig. 2d, His3 couldn’t successfully keep the expansion of coatings assembled LbL with TA, and the coating thickness considerably elevated with rising chain size. The thickest coating was ready utilizing His9. This is perhaps as a result of the variety of fees carried by the positively charged peptide elevated with rising chain size, which enhanced the interplay between the TA and peptide and, thus, elevated the movie thickness with rising chain size. Nonetheless, the coating wherein His12 was assembled with TA was barely thinner than that wherein His9 was assembled with TA. This is perhaps as a result of when the chain is just too lengthy, the potential resistance between the chain constructions may weaken the interplay between the TA and peptide, which decreases the movie thickness.
The impact of the peptide species on the thickness of the peptide coating was investigated by changing the amino-acid species within the positively charged peptide chain. As proven in Fig. 2e and f, the positively charged peptide (Lys6) was used as a template, and two positively charged Lys amino acids have been changed with two negatively charged Asp, uncharged Gly, or hydrophobic Ile or Phe amino acids and assembled with TA. Clearly, the coating comprising Lys6 assembled with TA was the thickest, and the coating wherein Lys4–Asp2 was assembled with TA grew the slowest. It’s because the cost densities of all of the peptides that have been substituted with negatively charged and uncharged and hydrophobic amino acids have been diminished to completely different levels in contrast with the unique positively charged peptide (Lys6). Specifically, the deprotonated substitution of negatively charged amino acids cancels the protonation-induced optimistic cost within the fundamental negatively charged chain, which considerably reduces the optimistic cost density on the peptide chain floor and, thus, slows the expansion of the LbL-assembled coating. Though uncharged amino-acid substitution might considerably improve the hydrogen-bonding interplay of the peptide with TA [41], the meeting of the peptide with TA continues to be a lot slower than that of the peptide with unsubstituted Lys6, indicating that hydrogen-bonding interactions play a lesser position than electrostatic interactions for assembling TA with peptides. Though hydrophobic amino-acid substitution may considerably enhance the hydrophobic interplay between the peptide and TA, the meeting of the peptide with TA was nonetheless considerably slower, indicating that hydrophobic interactions play a lesser position than electrostatic interactions for assembling TA with peptides. Due to this fact, for assembling TA with peptides LbL, electrostatic interactions are dominant. Notably, peptide coatings ready utilizing polyphenol constructing blocks present good substrate universality as a result of polyphenols readily adsorb on varied materials substrates [42]. As proven in Fig. 2g, polypeptide coatings have been ready on Ti, Si, PS, quartz, and glass substrates, and the substrate shade clearly modified from mild blue to mild yellow earlier than and after coating, respectively, indicating that coatings have been profitable deposited, the place mild yellow originates from TA.
(a) Meeting of various polyphenols with peptides (smiling and crying emoticons imply can and can’t be assembled, respectively). Results of (b) pH, (c) salt focus, (d) peptide chain size, and (f) amino-acid sorts on coating progress. (e) Schematic exhibiting substitution of two amino acids in Lys6 peptide. (g) Digital images of (TA–Lys6)15 coatings fabricated on Ti, Si, PS, quartz, and glass substrates
Stability is a crucial issue for organic functions of coatings [43]. DMEM comprises varied amino acids and glucose and is extensively used for culturing varied cells [44]. Coating stability below physiological circumstances will be understood by observing modifications within the thickness of coatings immersed in DMEM. As proven in Determine S13a, the coating thickness negligibly decreased with prolonged immersion in DMEM till the seventh day, indicating the nice stability of the coating below physiological circumstances. Moreover, the coating didn’t degrade when immersed in low (Determine S13b) and excessive (Determine S13c) concentrations of NaCl options, indicating that the coating has good salt resistance. As well as, the coating additionally reveals good urea resistance (Determine S13d). The nice stability of the coating is attributed partly to the nice charge-matching impact between the positively charged peptide and the negatively charged polyphenol, but in addition to the stronger interplay pressure supplied by the small-molecule meeting between the brief peptide and the small-molecule polyphenol. Notably, by individually immersing the as-prepared peptide coatings in numerous pH buffers, the coatings had vital pH-responsive properties. Though the coating reveals good stability below mildly acidic circumstances (pH 5, Determine S13e), it quickly decomposes below strongly acidic circumstances (pH < 3, Determine S13f). Due to this fact, the TA–peptide coating has good potential for software to acid-responsive drug carriers.
Thermodynamic mechanisms of TA and peptide meeting
Isothermal titration calorimetry was used to find out the thermodynamics of interactions between the peptide and TA and acquire binding thermodynamic parameters, comparable to enthalpy, entropy, and Gibbs free-energy modifications (ΔH, ΔS, and ΔG, respectively). For these measurements, TA options have been loaded into the injection syringe and titrated into completely different peptide options in pattern cells. The enthalpy change was monitored (Determine S14) after which plotted as a operate of the molar ratio of the peptide and TA (Fig. 3a–g). The experimentally measured titration enthalpy of the TA and peptide was obtained by subtracting the dilution enthalpy of the buffer–TA (Determine S14h) from that of the heart beat sign for the TA answer injected into the peptide answer (Determine S14 a–g). All of the evaluated peptides that complexed with TA confirmed a big change in enthalpy, indicating interplay and binding between the TA and peptide. The endpoint exotherms of the titration between TA and completely different peptides have been roughly 0, and all of the titration exotherms confirmed an excellent match with the analytical mannequin (Fig. 3a–g), indicating saturated binding between molecules and believable information outcomes. The fitted thermodynamic parameters (ΔH, ΔG, and − TΔS) obtained by titrating TA with completely different peptides are listed in Fig. 3h. All of the evaluated peptides that complexed with TA confirmed a destructive ΔG, indicating that the affiliation was spontaneous. Nonetheless, ΔH and − TΔS have been considerably completely different for the TA and completely different peptides, indicating completely different driving forces and interactions. Binding between positively charged peptides (Arg6, His6, and Lys6) and TA was predominantly enthalpy pushed with a excessive ΔH and an unfavorable entropic contribution, implying that enthalpic and entropic contributions equilibrated. The meeting of the negatively charged peptide (Asp6) with TA, alternatively, was codriven by entropy and enthalpy and since |−TΔS| > |ΔH|, binding was dominated by entropy. In distinction, Professional6–TA and Tyr6–TA bindings confirmed an unfavorable enthalpy and a good entropy and have been, subsequently, entropy pushed, which is according to hydrophobic affiliation. Evaluation of the ITC information signifies that though a number of interactions might exist between the TA and peptides, the enthalpy drive supplied by electrostatic interactions is critical to maintain the expansion of LbL-assembled coatings of polyphenols and peptides. As well as, thermodynamic modifications within the binding between the TA and positively charged His6 have been investigated below acidic circumstances (pH 3). The numerous lower in |ΔH| at pH 3 relative to pH 7 signifies that the electrostatic interplay between the TA and His6 is severely weakened below acidic circumstances and results in the decomposition of the assembled coating at pH 3. Due to this fact, positively charged peptides are an appropriate selection for meeting with polyphenols to organize multilayered coatings.
Fitted curves for modifications in molar enthalpy plotted as operate of peptide-to-TA molar ratio obtained by titrating (a) Arg6, (b) His6, (c) Lys6, (d) Asp6, (e) Professional6, (f) Tyr6, and (g) His6 in TA at 25 °C and pH 3. (h) Abstract of thermodynamic parameters (ΔH, ΔG, and − TΔS) decided from ITC evaluation
Molecular dynamics simulation of TA and peptide meeting
To additional examine the interplay between TA molecules and peptides, a sequence of all-atom molecular dynamics (MD) simulations have been carried out. As proven in Fig. 4a-c, initially (0 ns), the TA molecules and predicted peptides have been positioned randomly in a water field of 12.7 × 12.7 × 12.7 nm [3]. In about 90 ns from the all-atom MD simulation trajectory, a lot of the TA molecules aggregated and fashioned one main cluster. In 180 ns from the all-atom MD simulation trajectory, all of the TA molecules aggregated and fashioned one cluster, the peptides are distributed across the TA molecules and work together with the TA cluster. That is according to the peptide-coated floor noticed by LP-AFM and explains the rationale why the TA with His6 meeting coating consists of particles. Subsequently, we carried out a sequence of 500 ns all-atom MD simulations on the results of an acidic answer setting on the interplay between the peptide composed of six histidine residues and TA molecules. The construction of the peptide composed of six histidine residues (His6 peptide) was predicted by AlphaFold2. In Fig. 4d, the imply sq. deviation (MSD) curve of His6 peptide and TA molecules within the acid (pH = 3) and impartial (pH = 7) answer setting within the 500 ns all-atom MD simulation confirmed that the TA molecules aggregated and fashioned one main particle in about 200 ns time scale. Just one His6 peptide was captured alongside the floor of aggregated TA molecules (Fig. 4e) and the MSD curve indicated that the His6 peptide tends to diffuse and depart from TA molecules within the acid (pH = 3) answer setting. Then again, a sequence of His6 peptides have been captured alongside the floor of the aggregated TA molecules (Fig. 4f) and the MSD curve indicated that the His6 peptide tended to work together with TA molecules within the impartial (pH = 7) answer setting. That is according to the experimental outcomes (Fig. 2b).
(a–c) The snapshot (0 ns, 90 ns, 180 ns) from the all-atom molecule dynamics simulation trajectory confirmed the aggregation of TA molecules and peptides. The TA molecules have been proven in licorice coloured and the peptides have been proven in new cartoon and silver. (d) The MSD time evolution of His6 peptide (dashed) and TA molecules (strong) within the acid (pH = 3, blue) and impartial (pH = 7, pink) answer setting. (e) The interplay between His6 peptide (inexperienced) and TA molecules (magenta) within the acid (pH = 3) answer setting. (f) The interplay between His6 peptide (inexperienced) and TA molecules (magenta) within the impartial (pH = 7) answer setting. The atoms which will fashioned hydrogen bonds have been proven within the yellow dashed line
Moreover, the constructions of the positively charged peptides (Lys6 and Arg6 peptide) and the negatively charged peptide (Asp6 peptide) have been predicted by AlphaFold2, respectively, and a sequence of 500 ns all-atom MD simulations have been carried out to review their interactions with TA molecules. In Determine S15a, the imply sq. deviation (MSD) evolution of Lys6, Asp6, and Arg6 peptide and TA molecules in a impartial (pH = 7) answer setting within the 500 ns all-atom MD simulation confirmed that the TA molecules aggregated and fashioned one main particle in about 300 ns time scale. A number of positively charged Lys6 and Arg6 peptides can subsequently be captured alongside the floor of the aggregated TA molecule, whereas just one negatively charged Asp6 peptide will be captured (Determine S15b-d). In the meantime the MSD curves indicated that positively charged peptides (Lys6 and Arg6 peptide) tended to be captured by the aggregated TA molecules whereas negatively charged peptides (Asp6 peptide) tended to diffuse and detach from the TA molecules. As well as, molecular dynamics simulations confirmed stronger interactions between TA and positively charged peptides relative to negatively charged peptides. That is according to the experimental outcomes and illustrates the significance of electrostatic interactions for polyphenol-peptide meeting.
Owing to the complexity of protein constructions and unclear interplay areas, interactions between proteins and polyphenols have all the time been troublesome to elucidate. For instance, earlier research have demonstrated electrostatic, hydrogen-bonding, and hydrophobic interactions between the lysozyme (Lyz) and TA [45]. Lately, we have now discovered no covalent interactions throughout the meeting of TA and Lyz within the pH 6–8 vary, and though the driving pressure for assembling each originates from favorable enthalpy modifications as a result of noncovalent interactions, the precise interplay species from which the enthalpy modifications originate continues to be unknown [26]. Interactions and thermodynamic processes between TA and peptides containing completely different particular sequences and exhibiting completely different properties allow a deeper understanding of the motion mechanism between the lysozyme and TA. This examine reveals that though all sorts of peptides (charged, uncharged, and hydrophobic) interacted with TA, the favorable enthalpy change as a result of electrostatic interactions was the primary driving pressure for sustaining the meeting of the peptide and TA coatings, and the favorable enthalpy change as a result of positively charged peptides (Arg6, His6, and Lys6) was a lot bigger than that as a result of negatively charged peptides (Asp6). Equally, though all areas of Lyz (charged, uncharged, and hydrophobic) can concurrently exhibit a number of noncovalent interactions with TA, electrostatic interactions dominate between the positively charged areas (amino-acid sequences) and TA, and favorable enthalpy modifications as a result of electrostatic points of interest are the primary purpose for sustaining the expansion of Lyz–TA movies LbL, which clearly reveals the motion mechanism throughout the LbL meeting of Lyz and TA and offers a brand new thought for understanding and regulating the interplay mechanism between proteins and polyphenols.
Cell proliferation and antioxidant results of peptide coatings
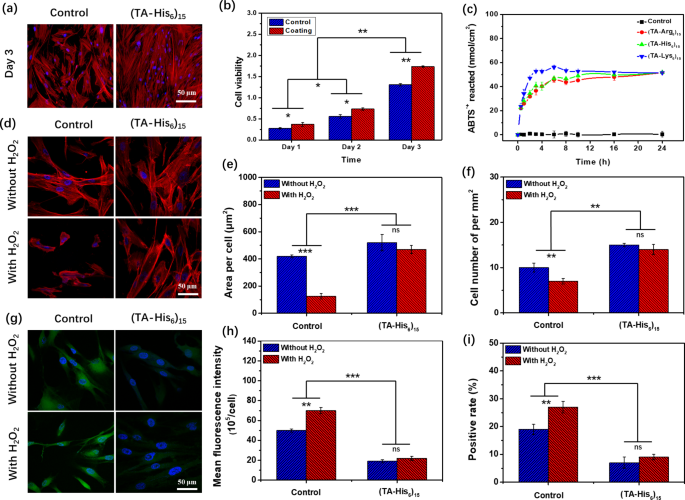
Peptide coatings exhibit good hydrophilicity and, thus, present appropriate circumstances for cell progress (Determine S16). Determine 5a and b present the graphical and quantitative analyses of the impact of the coating wherein TA was assembled with the positively charged peptide (His6) on cell proliferation, respectively. Proliferation was noticed for dental pulp stem cells cultured on (TA/His6)15-coated and management clean slides. Determine 5a reveals the fluorescence microscopy pictures after 3 d of cell tradition. The dental pulp stem cells grew effectively on each the management clean and (TA/His6)15-coated slides, and the cells cultured on the (TA/His6)15-coated slide have been denser and extra elongated than the cells cultured on the management slide, which signifies that the coating helped to distinguish the stem cells. On the (TA/His6)15 coating, mobile proliferation was quantified utilizing the CCK-8 technique to rely the variety of cells, as proven in Fig. 5b. Though the cell viability elevated with extended incubation on each the management and (TA/His6)15-coated slides, the cells grew extra quickly on the coating and have been considerably completely different from the cells that have been cultured on the management slide, indicating that the (TA/His6)15 coating was useful for selling cell proliferation. Though we hypothesized that the proliferative operate of the coating may originate from the formation of a practical secondary construction after the peptide meeting, CD spectroscopy didn’t reveal any apparent peptide secondary construction earlier than or after meeting (Determine S17). Due to this fact, the proliferative operate was primarily derived from the exercise and interplay between the TA and peptides.
Determine 5c reveals the ABTS•+ elimination kinetic curves obtained for varied coatings wherein TA was assembled with completely different positively charged peptides at pH 7. Relative to the clean management, TA/peptide-coated samples confirmed vital ABTS•+ radical scavenging. Though the ultimate quantities of free-radical scavenging have been related for various coatings, the free radical–scavenging charges have been considerably completely different, which can be brought on by completely different interplay strengths between the TA and completely different positively charged peptides. Interactions between TA and Arg6, His6, and Lys6 step by step weakened, which accelerated the free-radical scavenging of the corresponding coatings. To additional consider the intracellular antioxidant skill of the coatings, H2O2 was used to stimulate ROS formation as a result of H2O2 can quickly generate appreciable ROS. As proven in Fig. 5d, in contrast with the nontreated cells, the H2O2-treated cells cultured on the glass slide (within the management group) have been drastically diminished in each dimension and quantity, whereas the cells cultured on the (TA–His6)15-coated slide negligibly modified in each dimension and quantity. Statistical evaluation of the variety of cells and mobile space additional confirmed these observations, as displayed in Fig. 5e and f, respectively. These outcomes recommend that the (TA–His6)15 coating has sturdy antioxidant exercise.
To visualise the extent of intracellular ROS, cells on slides and coatings have been stained (inexperienced) utilizing an ROS detector and imaged earlier than/after remedy with H2O2. As proven in Fig. 5g, cells cultured on clean slides have been greener than these cultured on the (TA/His6)15 coating each earlier than and after H2O2 remedy. Cells cultured on clean slides grew to become brighter after H2O2 remedy, whereas the colour of cells cultured on the (TA/His6)15 coating negligibly modified. The imply fluorescence depth of every cell was analyzed utilizing ImageJ software program, as proven in Fig. 5h. The imply fluorescence depth of every cell considerably elevated for cells cultured on clean slides and negligibly modified for cells cultured on the (TA/His6)15 coating. To detect additional variations in intracellular ROS ranges, stream cytometry, which is extra delicate to fluorescence depth, was used, and the proportion of optimistic cells was calculated and analyzed utilizing the accent software program, as proven in Fig. 5i. After H2O2 remedy, the proportion of optimistic cells elevated in all of the samples. Nonetheless, cells cultured on clean slides confirmed a a lot increased ploidy than these cultured on the (TA/His6)15 coating. These outcomes present that the (TA–His6)15 coating has good ROS resistance. Notably, (TA/Lys6)15 coating ready by the identical technique exhibited higher cell proliferation (Determine S18) and mobile antioxidant results (Determine S19), which supplied a superior service for subsequent practical customization of the peptide coating, additional validating the universality of the polyphenolic peptide practical coating preparation technique.
(a) Fluorescence microscopy pictures of dental pulp stem cells seeded on glass and (TA/His6)15-coated slides for 3 d. (b) Relative actions of dental pulp stem cells seeded on glass and (TA/His6)15-coated slides for 1, 2, and three d. (c) Time evolution for amount of ABTS•+ reacted with (TA/hexapeptide)15 coating. (d) Fluorescence microscopy pictures of dental pulp stem cells seeded on (TA/His6)15-coated slides earlier than and after H2O2 remedy. (e) Space per cell and (f) variety of cells per mm [2] on (TA/His6)15-coated slides earlier than and after H2O2 remedy. (g) Fluorescence microscopy pictures of ROS detector inside dental pulp stem cells seeded on (TA/His6)15-coated slides earlier than and after H2O2 remedy. (h) Imply fluorescence depth per cell for cells seeded on (TA/His6)15-coated slide earlier than and after H2O2 remedy. (i) Constructive fee of intracellular ROS ranges generated primarily based on cytometry information
Multilayered capsules assembled from TA and peptides
As proven in Fig. 6a, PS was used as a template, and multilayered particles have been ready utilizing TA and Lys6 constructing blocks and LbL meeting. Then, the template was eliminated utilizing THF to acquire multilayered capsules. The floor potential and particle dimension of the multilayered particles and capsules have been monitored throughout LbL meeting (Fig. 6b). Zeta potential modifications obtained throughout the multilayered-capsule preparation confirmed that earlier than the LbL meeting, the PS template had a destructive potential, which decreased after TA deposition and elevated after Lys6 deposition owing to destructive and optimistic fees of TA deprotonation and Lys6 protonation at pH 7, respectively. This means that the LbL meeting of TA and Lys6 was pushed by electrostatic interactions. As a result of the negatively charged TA content material was increased than the positively charged Lys6 content material within the LbL movie, the potential continued to lower with rising variety of coating layers deposited on the multilayer particle floor, which is according to the QCM-D and XPS outcomes. The multilayered capsules had a better potential than the multilayered particles, indicating that the template was utterly eliminated. Throughout the multilayered-capsule preparation, the particle-size variation confirmed that particles grew with rising variety of assembled layers, indicating that TA and Lys6 assembled LbL on the template floor. The particles shrank throughout each the LbL meeting on the template and the template elimination. The obtained multilayered capsules may break or disintegrate throughout ultrasonication in a strongly acidic setting and, thus, launch their constituents and inside substances (Fig. 6c). Intact capsules (Fig. 6d) broke (Fig. 6e) below weak sonication, indicating that the assembled capsule layer had inflexible mechanical properties. A transparent Tyndall phenomenon (Fig. 6f) was noticed by shining a light-weight beam into the capsule dispersion, indicating the presence of steady intact capsules. When the answer pH was adjusted to under 3, the Tyndall phenomenon disappeared (Fig. 6g), indicating the decomposition of the capsules. These outcomes present that multilayered capsules assembled with the TA and peptides had a big acidic pH response, which endowed them with potential for sensible software as drug carriers.
(a) Schematic exhibiting preparation of multilayered capsules by LbL meeting. (b) Zeta potential and particle-size variations throughout multilayered-capsule preparation. (c) Schematic exhibiting multilayered-capsule decomposition by ultrasonication or pH stimulation. SEM morphology of multilayered capsules (d) earlier than and (e) after ultrasonication stimulation. Tyndall phenomenon in multilayered-capsule options (f) earlier than and (g) after low pH stimulation
RGD-sequence-modified cell adhesion-promoting and osteogenic peptide coatings
Functionalized coatings will be ready by modifying practical sequences on peptide molecule surfaces. Reportedly, the RGD sequence can strongly stimulate and promote cell adhesion and progress [46]. Thus, Lys6 was used as a positively charged peptide template to confer adhesion properties to the coating and promote osteogenic mobile differentiation by modifying the RGD sequence (Fig. 7a). Early adhesion and diffusion of osteoblasts are the keys to good osseointegration. After Mc3t3-E1 cells have been inoculated on materials surfaces for two and 4 h, the early adhesion of Mc3t3-E1 cells on glass (management) and (TA–Lys6)6– and (TA–Lys6/RGD)6-coated slides have been noticed utilizing fluorescence microscopy. As proven in Figs. 2h and 7b after inoculation, cells have been clearly subtle on the (TA–Lys6/RGD)6 floor. In contrast with the cell morphology at 2 h, the cell morphology at 4 h revealed that cells have been additional unfold in all of the samples. Specifically, the (TA–Lys6/RGD)6 morphology was clear and resembled that of osteoblasts. The statistical analyses of the variety of cells and cell space are proven in Fig. 7c and d, respectively. At 2 and 4 h, though the cells cultured on the (TA–Lys6)6-coated and management glass slides have been negligibly completely different, the variety of cells cultured on the (TA–Lys6/RGD)6-coated slides was considerably increased, and the cell space was considerably bigger, indicating that the cells cultured on the (TA–Lys6/RGD)6 coating confirmed higher early adhesion, which was attributed to the modification of the practical RGD sequence. Subsequently, cell adhesion was evaluated by shaking cells cultured on completely different substrates for a sure interval after which evaluating cell viability. As proven in Fig. 7e, after the cells have been shaken, the relative cell viability was elevated on each the (TA–Lys6)6 and (TA–Lys6/RGD)6 coatings as a result of cells have been considerably shed from the clean substrate. Furthermore, the relative cell viability was considerably increased on the (TA–Lys6/RGD)6 coating than on the (TA–Lys6)6 coating, indicating that the coating assembled utilizing RGD-sequence-modified peptides enhanced cell adhesion.
As a result of MC3T3-E1 cells may promote osteogenic differentiation and bone matrix mineralization throughout osseointegration [47], the osteogenic properties of various peptide coatings have been investigated. Alkaline phosphatase (ALP) is an osteoblast exoenzyme, and its expression exercise is a particular function of osteogenic differentiation, which is a crucial indicator of early osteoblast differentiation [48]. ALP staining was carried out on the seventh and 14th days of cell tradition and noticed below a optimistic microscope (Fig. 7f). For all of the coatings, the ALP exercise was low on day 7, particularly the presence of quite a few unstained osteoblasts on the management and (TA–Lys6)6-coated slides, whereas the (TA–Lys6/RGD)6 coating confirmed comparatively extra shade and deeper staining. With extended incubation, the ALP exercise elevated on day 14 in comparison with day 7, and the staining sample on the (TA–Lys6/RGD)6 coating was dense blue–purple, which was considerably higher than that of the management and (TA–Lys6)6 coating, indicating that the (TA–Lys6/RGD)6 coating was superior for selling osteogenic differentiation. Alizarin pink can react with calcium nodules, and the impact of osteogenesis-related extracellular matrix mineralization will be confirmed utilizing alizarin pink staining after it’s induced and cultured in vitro [49]. As a result of extracellular matrix mineralization occurred late, alizarin pink staining was carried out on the 14th and twenty first days of cell tradition and noticed below a optimistic microscope (Fig. 7g). On the 14th day, the experimental and management teams have been negligibly completely different, and no apparent calcium nodules have been noticed within the experimental group. On the twenty first day, though calcium nodules have been nonetheless not noticed within the management and (TA–Lys6)6 teams, the staining was considerably deeper than that on the 14th day. The floor of the (TA–Lys6/RGD)6 movie was dyed reddish, and some calcium nodules appeared, indicating that (TA–Lys6/RGD)6 may promote matrix mineralization.
To grasp osseointegration on the coating floor extra deeply, the expression of osteogenic markers was analyzed utilizing qRT–PCR at completely different phases of differentiation. Kind I collagen (COL-1) is an extracellular matrix protein that may stimulate osteoblast adhesion and differentiation. Alkaline phosphatase (ALP) is a typical protein produced throughout osteoblast differentiation, and ALP exercise usually represents the early phenotypic marker of osteoblast formation. Osteocalcin (OCN) seems on the finish of osteogenic differentiation and binds to Ca2+ ions to manage bone mineralization by sustaining or regenerating bone tissue. Runx2 is the central management gene of the osteoblast phenotype, which controls the deposition of extracellular components of sort I collagen and may successfully regulate osteoblast differentiation [50]. After the cells have been cultured on the coating surfaces for 14 days, osteoblast differentiation markers COL-1, ALP, OCN, and Runx2 have been analyzed utilizing RT-PCR (Fig. 7h). The (TA–Lys6/RGD)6 coating considerably upregulated the expression of osteogenesis-related genes in comparison with the management and (TA–Lys6)6 coating. These outcomes present that the (TA–Lys6/RGD)6 coating can obtain a wonderful osteogenic impact within the early and center phases of osteogenesis, which offers an necessary assure for osseointegration.
(a) Schematic of methods for modifying peptides utilizing RGD sequences for making ready cell adhesion-promoting and osteogenic peptide coatings. (b) Fluorescence microscopy pictures of Mc3t3-E1 cells seeded on glass and (TA–Lys6)6– and (TA–Lys6/RGD)6-coated slides for two and 4 h. (c) Variety of Mc3t3-E1 cells per mm [2] and (d) per cell space on completely different coatings at 2 and 4 h. (e) Relative cell viabilities on (TA–Lys6)6 and (TA–Lys6/RGD)6 coatings earlier than and after shaking for 30 min. (f) Mc3t3-E1 cells have been inoculated and cultured on glass and (TA–Lys6)6– and (TA–Lys6/RGD)6-coated slides and ALP stained on seventh and 14th days. (g) Mc3t3-E1 cells have been cultured on completely different coatings for 14 and 21 days, and alizarin pink staining was used to point bone mineralization. (h) Expression ranges of differentiation-related genes COL-1, ALP, OCN, and RUNX2 after culturing Mc3t3-E1 cells on glass and (TA–Lys6)6– and (TA–Lys6/RGD)6-coated slides for 14 days
CM15- and PEG-sequence-modified antimicrobial and antifouling peptide coatings
Antimicrobial and antifouling features are sometimes required for medical diagnostic units and therapeutic instruments/implants. [51] Due to this fact, CM15 sequences, which have antimicrobial properties [52], and PEG chain segments, which have antifouling properties [53], have been used to switch the surfaces of positively charged peptides (Lys6). Then, peptide coatings have been ready, and their antimicrobial and antifouling properties have been investigated.
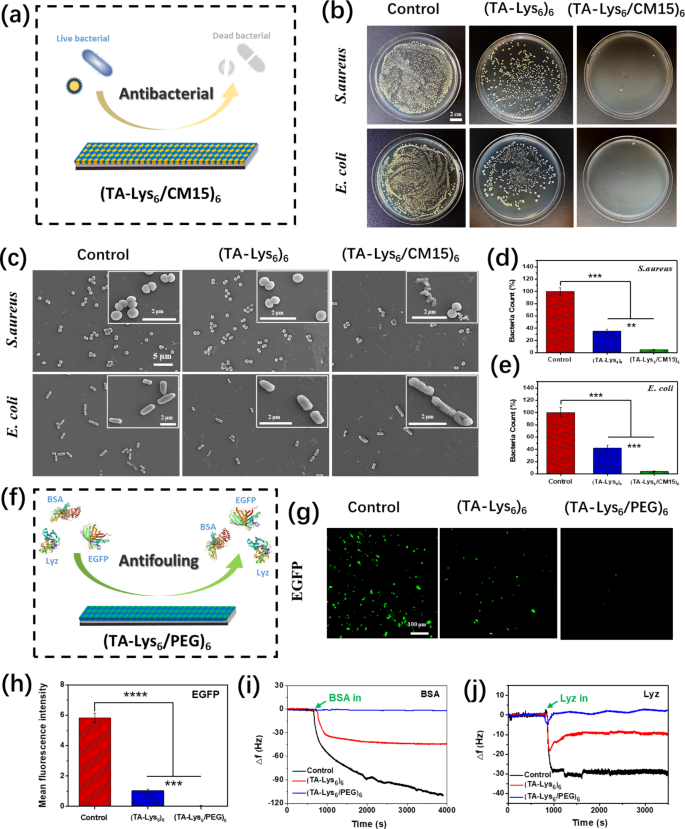
The (TA–Lys6/CM15)6 coating was obtained by assembling Lys6/CM15 peptide with TA by a LbL meeting technique, and its antimicrobial properties have been subsequently investigated utilizing S. aureus and E. coli (Fig. 8a). Determine 8b reveals the expansion of bacterial suspensions on agar media earlier than and after contact with the completely different coatings. Quite a few micro organism within the suspensions contacted the management group and (TA–Lys6)6 coating, whereas virtually no bacterial progress was noticed within the suspensions contacted with the (TA–Lys6/CM15)6 coating. Subsequently, the quantity and morphology of micro organism on completely different coatings have been noticed utilizing SEM. As proven in Fig. 8c, considerably fewer micro organism grew on the (TA–Lys6/CM15)6 coating than on management group floor and (TA–Lys6)6 coating. Furthermore, on the management group floor and (TA–Lys6)6 coating, S. aureus and E. coli cells have been easy and intact, whereas micro organism on the (TA–Lys6/CM15)6 coating have been shrunken and ruptured. Upon additional evaluation, the variety of surviving micro organism was evaluated for suspensions in touch with the completely different coatings, as proven in Fig. 8d and e. Apparently, the odds of S. aureus and E. coli bacterial counts on the (TA–Lys6)6 coating decreased in comparison with the management group, whereas the odds of S. aureus and E. coli bacterial counts on the (TA–Lys6/CM15)6 coating additional decreased in comparison with the (TA–Lys6)6 coating, indicating that the (TA–Lys6/CM15)6 coating has glorious antimicrobial properties. The superior antimicrobial property of the (TA–Lys6/CM15)6 coating relative to the (TA–Lys6)6 coating and management group means that the antimicrobial sequence (CM15) loaded on the positively charged peptide (Lys6) retained the exercise and exhibited glorious antimicrobial properties after being assembled with polyphenols in a multilayer coating.
The (TA–Lys6/PEG)6 coating was obtained by assembling Lys6/PEG peptide with TA by a LbL meeting technique, and its antifouling properties have been subsequently investigated (Fig. 8f). The amount or high quality of proteins adsorbed on a coating floor over a particular time can be utilized to measure the antifouling properties of coatings [9]. Determine 8g and h present the adsorption of EGFP protein on completely different coatings, which was additional analyzed utilizing quantitative fluorescence evaluation. The outcomes confirmed that considerably much less EGFP adsorbed on the (TA–Lys6)6 coating than on the management group floor, whereas even much less EGFP adsorbed on the (TA–Lys6/PEG)6 coating. Subsequently, the adsorption of each proteins (BSA and Lyz) on completely different coatings was investigated utilizing QCM-D (Fig. 8i and j), whereby a lower in Δf signifies a rise within the mass deposited on the quartz crystal chip. The outcomes confirmed adsorption according to EGFP, i.e., the standard of protein adsorbed on the (TA–Lys6/PEG)6 coating, was considerably decrease than that on the (TA–Lys6)6 coating and management floor, which indicated that the (TA–Lys6/PEG)6 coating confirmed glorious antifouling properties. The superior antifouling property of the (TA–Lys6/PEG)6 coating relative to the (TA–Lys6)6 coating and management group means that antifouling sequences (PEG) loaded on positively charged peptides (Lys6) exhibit antifouling properties which are maintained after being assembled with polyphenols in a multilayered coating.
Antibacterial properties of coatings: (a) Schematic of methods for modifying peptides utilizing CM15 sequences for making ready antibacterial peptide coatings. (b) Digital {photograph} and (c) SEM pictures of S. aureus and E. coli on clean floor and (TA–Lys6)6 and (TA–Lys6/CM15)6 coatings. Percentages of dwell (d) S. aureus and (e) E. coli counted from colony space in digital {photograph}. Antifouling properties of coatings: (f) Schematic of methods for modifying peptides utilizing PEG sequences for making ready antifouling peptide coatings. (g) Fluorescence microscopy pictures of EGFP on clean floor and (TA–Lys6)6 and (TA–Lys6/PEG)6 coatings. (h) Imply fluorescence depth of EGFP counted from fluorescence micrographs. Frequency shifts (Δf) of clean floor and (TA–Lys6)6 and (TA–Lys6/PEG)6 coatings immersed in (i) BSA and (j) Lyz protein options