Characterization of coatings
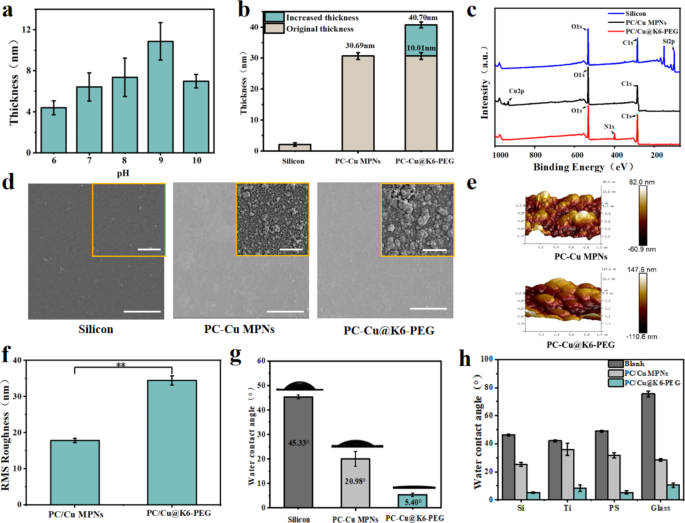
The PC-Cu MPNs coatings have been ready by self-assembly within the aqueous section, as beforehand reported [31]. Subsequently, K6-PEG was deposited onto the MPNs coatings by immersing MPNs into K6-PEG answer (Fig. 1a). As illustrated in Fig. 2a, the thickness of deposited K6-PEG elevated with the elevated with the rise in pH of the K6-PEG answer earlier than pH 9, then decreased at pH 9. The pH 9 was chosen for the deposition of K6-PEG within the following experiments. At pH 9, the change in coating thickness from 30.69 ± 2.13 nm to 40.70 ± 2.52 nm was noticed (Fig. 2b), suggesting a rise of about 10.1 nm after deposition of K6-PEG. Compared to the PC-Cu MPNs coatings, N1s alerts have been noticed on the PC-Cu@K6-PEG, whereas the nitrogen atoms within the coating are solely current in K6 (hexameric lysine), which supplies compelling proof for the profitable deposition of K6-PEG (Fig. 2c). The proportion of nitrogen atoms in PC-Cu@K6-PEG was about 8.23%. In distinction, the proportion of Cu atoms in PC-Cu MPNs underwent a notable decline, from 1.98 to 0.19% (Desk S1). These findings present additional proof on the profitable deposition of K6-PEG onto MPNs coatings. As a way to visualize the morphological adjustments of the coatings, the coatings have been deposited on silicon substrates and noticed utilizing a SEM. As illustrated in Fig. 2d, the floor of the PC-Cu MPNs coating gave the impression to be shaped by the successive aggregation of quite a few nanoparticles, whereas bigger measurement was noticed after the deposition of K6-PEG. As a way to higher perceive the floor profile, the morphology and roughness of the PC-Cu MPNs and PC-Cu@K6-PEG coatings have been examined by AFM. A wavy floor was noticed, just like the morphology noticed by SEM, and it may be seen that the particles of the PC-Cu@K6-PEG coatings are bigger than these of the PC-Cu MPNs coatings, and never solely that, the floor of the PC-Cu@K6-PEG coatings can also be wavy (Fig. 2e). As well as, in comparison with the PC-Cu MPNs coatings, the PC-Cu@K6-PEG coating exhibited a notable alteration in roughness, the foundation imply sq. (RMS) roughness elevated from 17.84 ± 0.59 nm to 34.46 ± 1.25 nm (Fig. 2f), according to the findings by the SEM (Fig. 2d). The WCA was decided earlier than and after K6-PEG deposition, because of the excessive sensitivity of WCA to floor variations. It was noticed that the WCA of the uncoated silicon substrate was 45.33 ± 0.75 °, whereas the WCA of the floor with PC-Cu MPNs was 20.98 ± 2.98 °. The WCA was additional diminished to five.40 ± 0.2 ° within the following deposition of K6-PEG (Fig. 2g), which strongly supported the efficiently deposition of K6-PEG. It’s noteworthy that the extraordinarily low WCA of PC-Cu@K6-PEG permits the formation of a sturdy hydration layer on the floor of the fabric, which in flip reveals a excessive potential to withstand biofouling. Moreover, the coating’s skill to be assembled on a spread of substrates, together with silicon wafers, titanium, glass, and PS plastic, was demonstrated (Fig. 2h), to show the benefit for its extensive utility. The WCA earlier than and after coating with PC-Cu MPNs or PC-Cu@K6-PEG was measured. The WCA of varied substrates with the PC-Cu MPNs coating diminished to twenty–30 °, and additional vital discount of the PC-Cu@K6-PEG coated surfaces have been noticed (< 10 ° and even < 5 ° for some substrates).
(a) The impact of pH worth on the thickness of K6-PEG on PC-Cu MPNs was investigated (PC-Cu MPNs have been immersed in K6-PEG (1 mg/ml) answer at pH 6, 7, 8, 9 and10 for twenty-four h); (b) The thickness of PC-Cu MPNs and PC-Cu@K6-PEG (PC-Cu MPNs immersed in K6-PEG (1 mg/ml) for twenty-four h, pH = 9); (c) XPS spectra of the clean silica substrate, PC-Cu MPNs and PC-Cu@K6-PEG; (d) SEM pictures of clean silicon substrates, PC-Cu MPNs coatings, and PC-Cu@K6-PEG coatings; (e) Floor morphology and (f) Root-mean-square roughness of PC-Cu MPNs and PC-Cu@K6-PEG coatings (measured by AFM); (g) Static WCAs of clean silicon substrates, PC-Cu MPNs, and PC-Cu@K6-PEG; (h) WCAs of silicon, titanium, glass and plastic substrates earlier than and after PC-Cu MPNs and PC-Cu@K6-PEG coatings. The size bars in (d) have been 500 nm and 25 μm
Mechanical properties and hydrophilic ageing of coatings
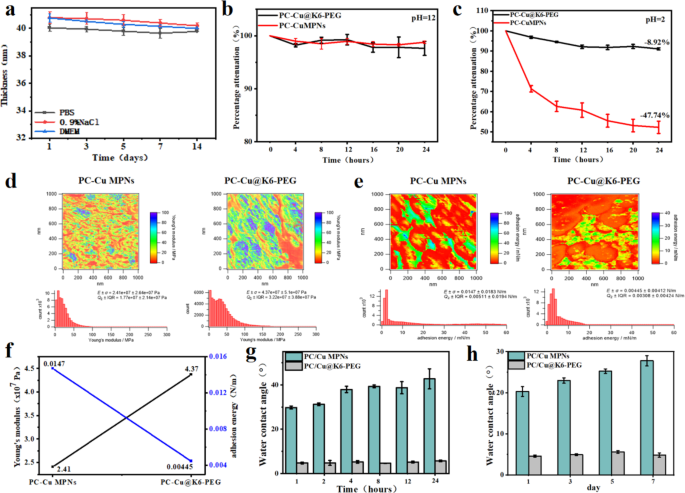
As an anti-biofouling coating on biomedical gadgets, it’s important that it maintains good stability beneath physiological circumstances for an prolonged time period. Due to this fact, adjustments in coating thickness over time have been monitored by immersing the PC-Cu@K6-PEG coatings in PBS, 0.9% NaCl answer and cell tradition medium DMEM (Fig. 3a). The outcomes confirmed that there was no vital lower within the thickness of the PC-Cu@K6-PEG coatings, suggesting that the PC-Cu@K6-PEG coating was steady within the above answer. It has been reported that MPNs lack long-term stability, particularly beneath harsh surroundings, corresponding to excessive acidic circumstances [32], which has restricted the appliance of MPNs. To check whether or not the introduction of K6-PEG might enhance this property, the PC-Cu MPNs coatings and PC-Cu@K6-PEG coatings have been subjected to immersion in NaOH (pH = 12) and HCl (pH = 2) options, respectively, for a interval of 24 h. As illustrated, each the PC-Cu MPNs and PC-Cu@K6-PEG coatings demonstrated glorious stability in a pH = 12 NaOH answer (Fig. 3b). Nevertheless, at pH 2, the thickness of the PC-Cu MPNs coatings decreased from the preliminary 30.5 ± 2.31 nm to fifteen.93 ± 3.05 nm (about 47.74% discount) inside 24 h, a steady lowering development was noticed over a 24 h interval (Fig. 3c). The PC-Cu@K6-PEG coating exhibited a notable lower in thickness from 30.5 ± 2.31 nm to 27.77 ± 0.52 nm (a discount of about 8.92%) inside the preliminary 12 h interval, adopted by a stabilization section. These knowledge demonstrated that the deposition of K6-PEG led to a major enhancement within the acid resistance, in comparison with the PC-Cu MPNs coating. The Younger’s modulus and adhesion vitality of the coatings have been decided by atomic power microscopy (AFM) previous to and following K6-PEG grafting. It may be noticed that the Younger’s modulus of PC-Cu MPNs is 2.41 × 10⁷ Pa, whereas following K6-PEG grafting, the Younger’s modulus will increase to 4.37 × 10⁷ Pa (Fig. 3d), representing an almost twofold enhance. Moreover, low floor vitality is intimately related to the anti-biofouling capability of the coating, and anti-biofouling coatings usually exhibit diminished floor adhesion vitality [33,34,35]. As illustrated in Fig. 3e-f, the floor adhesion vitality of the coating exhibited a notable decline from 0.0147 N/m to 0.00445 N/m following K6-PEG deposition. For superhydrophilic coatings, the power to keep up superhydrophilicity over time is essential for attaining efficient anti-biofouling efficiency. As a way to verify the impression of UV sterilization on biomedical functions, the coatings have been subjected to UV irradiation. The outcomes demonstrated that there was no notable alteration within the WCA on the coating floor following 24 h of UV irradiation (Fig. 3g). Moreover, no appreciable change within the WCA on the coating floor was noticed after the coating was immersed in PBS for 7 days (Fig. 3h). These findings recommend that the coating retains its preliminary superhydrophilicity, which is conducive to long-lasting antifouling efficiency.
(a) The impact of immersion time on the thickness of the fabric was investigated for 2 completely different solvents: phosphate buffered saline (PBS, 0.9% NaCl) and cell tradition medium (DMEM); The ablation of PC-Cu MPNs and PC-Cu@K6-PEG coatings was noticed following immersion in options with (b) pH = 12; (c) pH = 2; (d) Younger’s modulus pictures of coatings in a liquid surroundings and gaussian distribution of the coated Younger’s modulus; (e) Adhesion vitality pictures of PC-Cu MPNs and PC-Cu@K6-PEG coatings in a liquid surroundings and gaussian distribution of the coating adhesion vitality; (f) A comparability of the Younger’s modulus and adhesion vitality of PC-Cu MPNs and PC-Cu@K6-PEG coatings; (g) The WCAs of PC-Cu MPNs and PC-Cu@K6-PEG coatings after 1, 2, 4, 8, 12, and 24 h of UV irradiation; (h) The WCAs of PC-Cu MPNs and PC-Cu@K6-PEG coatings after 1, 3, 5, and seven days of immersion in PBS
Depositing mechanism of K6-PEG
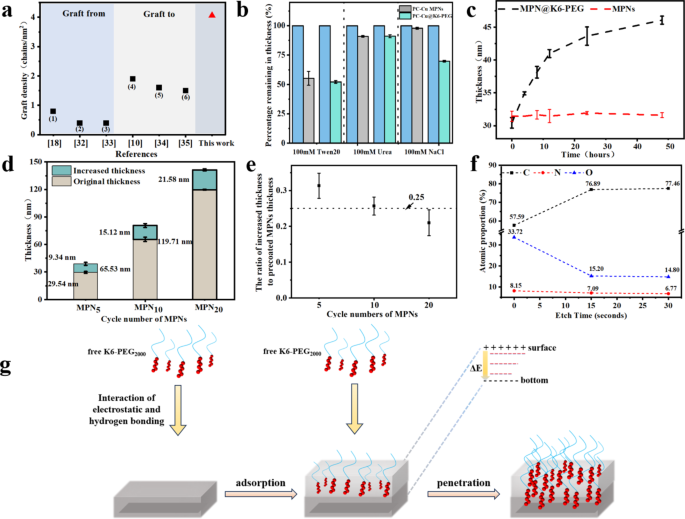
The fabrication of anti-biofouling polymer brushes represents the first technique of mitigating non-specific adhesion to medical system surfaces. The fabric composition and structural properties of polymer brushes are of great relevance to the prevention of biofouling. One of the crucial essential elements is the grafting density of polymer brushes. It has been demonstrated {that a} excessive graft density improve the structural stability, hydration energy (a stronger hydration layer), and resistance to bio-contamination of polymer brushes [36]. A comparability of the grafting densities of the polymer brushes obtained from the aforementioned strategies and the current work was illustrated in Fig. 4a [37,38,39,40,41,42]. The utmost grafting density reported in different paperwork was 0.79 chains/nm² for GF technique and 1.9 chains/nm² for GT technique. On this work, the calculated graft density of K6-PEG on PC-Cu MPNs was discovered to be 4.06 chains/nm2. Given these observations, an investigation was undertaken into the adsorption mechanism of K6-PEG in MPN coatings with a view to elucidating the rationale behind the elevated graft density noticed on the coating floor. By blocking a number of of those interactions, it’s potential to check the change in layer thickness of PC-Cu@K6-PEG with that of the MPNs management group, thereby enabling an investigation of the interplay between K6-PEG and MPNs. As illustrated in Fig. 4b, the coatings have been immersed in 100 mM Tween 20 (shielded from hydrophobic interplay), 100 mM NaCl (shielded from electrostatic interplay), and 100 mM urea (shielded from hydrogen bonding interplay), respectively. There was primarily no discount in MPNs within the NaCl answer. In distinction, the desorption price of PC-Cu@K6-PEG was 30%, suggesting that the deposition course of is primarily electrostatically pushed. This end result was according to the dependent deposition of K6-PEG on pH, which was a indication on electrostatic interplay [31]. Moreover, the deposited thickness of K6-PEG on MPNs is strongly relied on the immersing time (Fig. 4c). It confirmed very sluggish depositing course of with time, there was no plateau even after the examined 48 h. It was additional discovered that the deposition course of was additionally associated to the thickness of the preliminary MPN. As illustrated in Fig. 4d, for the MPNs with completely different cycle numbers and thickness of 29.54 ± 1.10 nm, 65.53 ± 2.35 nm, and 119.71 ± 0.57 nm, the elevated thicknesses have been 9.34 ± 1.61 nm, 15.12 ± 2.09 nm, and 21.58 ± 1.19 nm, respectively, after soaking into K6-PEG answer at pH 9 for twenty-four h (Fig. 4d). The elevated thicknesses attributed to K6-PEG deposition have been obtained as 9.34 ± 1.61 nm, 15.12 ± 2.09 nm, and 21.58 ± 1.19 nm for the MPNs respectively. To higher observe the contribution of precoated MPNs to the elevated thickness, the elevated thickness was normalized by the thickness of MPNs, and the information was confirmed in (Fig. 4e). It’s discovered that the ratio of elevated thickness to the thickness of precoated MPNs was very near 0.25, that means per 100 nm precoated MPNs contributed to a 25 nm enhance of K6-PEG coating.
(a) The graft density introduced by GF (symbols of (1–3)) and GT (symbols of (4–6)) methods in earlier paperwork, and this work; (1) PEG brushes shaped by surface-initiated aqueous-phase ATRP of methoxypolyethyleneglycol (750) acrylamide (MPEG350Am) monomers; (2) and (3) PEG brushes on silicon by floor initiated ATRP of oligo(ethylene glycol) methyl ether methacrylate (OEGMA300); (4) PEG layer immobilized on titanium by electrolytic discount utilizing o-quinone-linked PEG; (5) PEG brushes ready by adsorption of thiol-ended PEG onto gold surfaces; (6) PEG brushes fastened onto magnetite floor by the coordinative interplay between carboxyl-terminated PEG and iron; (b) Proportion remaining of PC-Cu@K6-PEG coatings abated after immersion in 100 mM urea, Tween 20 and NaCl options; (c) The impact of immersing time into K6-PEG and (d) the cycle numbers of PC-Cu MPNs on the thickness of PC-Cu@K6-PEG; (e) The ratio of the elevated thickness after K6-PEG deposition on MPNs with completely different cycle numbers to the thickness of the unique MPNs; (f) Atomic ratios of PC-Cu PC-Cu@K6-PEG after completely different etch instances (15 nm per etch depth with 15 s etching) measured by XPS; (g) Scheme of K6-PEG on the penetration means of PC-Cu MPNs
The fundamental distribution of PC-Cu@K6-PEG at various depths was subsequently examined via XPS etching. Particularly, the fundamental distribution on the floor of PC-Cu@K6-PEG was initially analyzed by XPS. Subsequently, the layer was etched downward as soon as (via a thickness of 15 nm) by XPS, sustaining the unique place. In the end, the layer was etched downward as soon as extra, additionally via a thickness of 15 nm, by XPS. The outcomes reveal that the floor of PC-Cu@K6-PEG movies was comprised of 57.59% carbon (C), 8.15% nitrogen (N), and 33.72% oxygen (O) atoms, respectively. Following the preliminary 15 s of etching, a lower within the share of detected N and O atoms was noticed, whereas the proportion of detected C atoms elevated. Following an extra 15 s of etching, the proportion of C, N, and O atoms within the movie remained at 76.89%, 7.09%, and 15.20%, respectively (Fig. 4f). Because the etching course of continues, the proportions of C, N, and O atoms are inclined to stabilize, with the atomic proportion of the N aspect remaining at 6.77%. These outcomes strongly indicated that the deposition of K6-PEG occurred not solely on the floor but additionally inside the MPN. The deposition means of K6-PEG is proposed in Fig. 4g. Initially, the free K6-PEG in answer is drawn to the MPN by electrostatic and hydrogen bonding interactions, ensuing within the adsorption of K6-PEG on the MPN. The optimistic cost of K6 modified the zeta potential on the MPN floor, thereby creating an electrostatic potential vitality distinction (ΔE) between the highest and backside of the MPN. Within the presence of this detrimental electrical area, the positively charged K6 is additional attracted deeper into the MPN till a even distribution of K6-PEG all through the MPN layer is achieved.
Passivated protein-adsorption properties
Contact of the interventional catheter with blood leads to the fast adsorption of plasma proteins (particularly fibrinogen) to the floor of the fabric, which then undergoes a conceptual transformation to type fibrin. The adsorption and denaturation of proteins can provide rise to a lot of problems, together with acute thrombosis, acute irritation, and a discount or lack of the organic operate of the floor. Consequently, it’s of paramount significance to evaluate the adsorption properties of proteins on the floor of implant supplies [43].
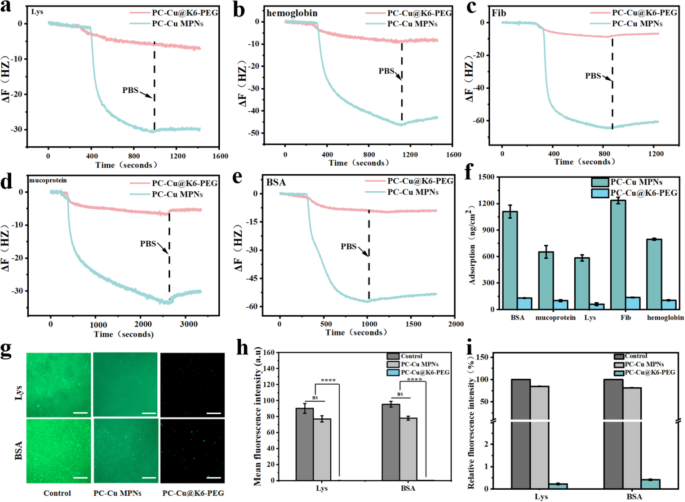
The non-fouling properties of hydrophilic supplies are intently associated to the hydration layer close to the floor. This tightly certain water layer varieties a bodily and energetic barrier that forestalls protein adsorption to the floor [44, 45]. As a way to decide the dynamic passivation adsorption capability of the coatings for various proteins, a sequence of checks have been carried out utilizing a quartz crystal microbalance (Q-CMD) to evaluate the adsorption of PC-Cu MPNs and PC-Cu@K6-PEG coatings for a spread of proteins. Proteins with disparate physicochemical properties (together with molecular weight (Mw) and isoelectric level (IP)) have been chosen for investigation (BSA has a molecular weight of 68 KD and IP 4.8; Lys has a molecular weight of 14–15 KD and IP 10.5–11.0; fibrinogen has a molecular weight of 330–340 KD and an IP 5.5; haemoglobin has a molecular weight of 67 KD and IP 4.5-5.0; and mucin has a molecular weight of 110 KD and IP 3.0–5.0). Particularly, 1 mg/ml BSA, Lys, fibrinogen, haemoglobin and mucin have been dissolved in 1X PBS (pH = 7.4), the gold coated sensor was mounted in a Q-CMD chamber, the peristaltic pump pace was 90 rpm and the system temperature was set at 25 ℃ PBS was then handed via till the curves grew to become smoother after which the PBS was modified to the protein options above. As illustrated in Fig. 5a-e, the time-dependent resonance frequencies on the gold sensor floor demonstrated that proteins have been adsorbed by flowing on the fabric floor. Compared to the PC-Cu MPNs group, the PC-Cu@K6-PEG coating group exhibited a considerably diminished change in frequency (6–9 instances decrease). As well as, the mass of proteins adsorbed on the floor of the gold sensor was calculated utilizing the equation beneath the viscoelastic mannequin, and the clean management group adsorbed essentially the most quantity of proteins on the floor as much as 1,235.32 ng/cm2, whereas the quantity of proteins adsorbed on the PC-Cu@K6-PEG was considerably diminished right down to a minimal of 4.09 ng/cm2 (Fig. 5f), and the anti-adsorption effectivity in opposition to proteins was 99.67%. These outcomes point out that the PC-Cu@K6-PEG coating has good antiadsorption properties for proteins with a large spectral vary.
In QCM-D, the plots of time-dependent resonance frequencies after the adsorption of (a) Lys, (b) hemoglobia, (c) Fib, (d) mucoprotein and (e) BSA on PC-Cu MPNs and PC-Cu@K6-PEG coatings; (f) The amount of the above adsorbed proteins on the PC-Cu MPNs and PC-Cu@K6-PEG coatings decided by Equations beneath the viscoelastic mannequin; (g) Confocal pictures of FITC-BSA and FITC-Lys adsorbed on glass (management) the PC-Cu MPNs and PC-Cu@K6-PEG coatings; (h) The imply fluorescent depth and (i) relative fluorescent depth of adsorbed FITC-BSA and FITC-Lys on glass (management) the PC-Cu MPNs and PC-Cu@K6-PEG coatings, as obtained from (g). The size bars in (g) have been 20 μm. (N = 3, no significance famous as “ns”, *p < 0.05, **p < 0.01, ***p < 0.001 utilizing t-test.)
Moreover, in an effort to extra successfully illustrate the coating’s capability to withstand protein adsorption, the samples have been incubated with 1 mg/mL of fluorescein isothiocyanate-labeled bovine serum proteins (FITC-BSA) and lysozyme (FITC-Lys) for a interval of 24 h. This allowed for a qualitative and semi-quantitative examination of the static adsorption behaviours of proteins on the floor of the supplies. As proven in Fig. 5g, a considerable amount of fluorescent protein adsorbed on the clean glass slice (management), leading to excessive floor fluorescence depth. In distinction, there was slight lower fluorescent sign on the PC-Cu MPN coatings and nearly no seen fluorescent sign on PC-Cu@K6-PEG coating. From the quantitative evaluation within the imply fluorescence (Fig. 5h), the quantity of fluorescence adsorbed on the floor of the PC-Cu@K6-PEG coating was considerably diminished, with a fluorescence depth of 0.5 a.u, which was diminished by an element of ∼ 150 in comparison with that of the PC-Cu MPNs and by an element of ∼ 200 in comparison with the clean glass (management). Determine 5i illustrated the relative fluorescence intensities by normalizing the samples’ depth with the management’s, through which a diminished relative fluorescence depth on PC-Cu MPN coating and vital discount on PC-Cu@K6-PEG coating was noticed. These outcomes steered that the coating had a robust passivation impact on protein adsorption.
Discount in bacterial adhesion behaviour
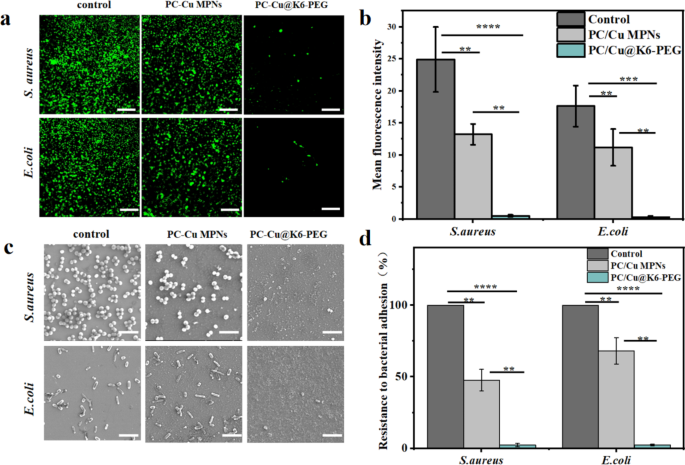
Earlier research have demonstrated {that a} vital proportion of micro organism include a wide range of protein appendages that play a direct or oblique position in adhesion [46], corresponding to flagella, hyphae, and coils [47,48,49]. Bacterial adhesion is often divided into three phases, commencing with reversible preliminary adhesion. That is adopted by a transition to enhanced irreversible adhesion, and eventually the event of a mature biofilm [50, 51]. Consequently, the inhibition of preliminary bacterial adhesion represents a pivotal technique for the prevention of biofilm formation. The preliminary 8 h following bacterial contact with the floor is considered the “decisive interval” for the prevention of bacterial adhesion. Consequently, the samples have been incubated with Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) for 8 h in an effort to examine their resistance to micro organism. Following an eight hours incubation interval, the micro organism have been stained with a LIVE/DEAD BacLight bacterial viability equipment (Steps supplied by provider) and noticed beneath a fluorescence microscope (Fig. 6a). It was noticed that extreme E. coli and S. aureus adhesion occurred on clean glass slice group (management) and the PC-Cu MPNs group. In distinction, just a few E. coli and S. aureus adhesion have been noticed on the floor of the PC-Cu@K6-PEG pattern. A semi-quantitative fluorescence evaluation (Fig. 6b) confirmed that the fluorescence depth of the PC-Cu MPNs group was barely diminished (by about two-fold) in comparison with the clean glass slides, whereas the typical fluorescence depth of the PC-Cu@K6-PEG group was diminished by about 50 folds in comparison with that of the clean glass slides group, and by about 25 folds in comparison with that of the PC-Cu MPNs. To offer a extra visible illustration of the coating’s resistance to bacterial adhesion, the incubated micro organism have been dehydrated and cured, after which noticed for bacterial adhesion on the pattern floor by SEM. As illustrated in Fig. 6c, a substantial variety of micro organism adhered to the wafer substrate (management group). In distinction, the variety of micro organism on the floor of PC-Cu MPNs was comparatively diminished, whereas a major discount in bacterial adherence was noticed on the floor of PC-Cu@K6-PEG. Moreover, the quantitative evaluation outcomes (Fig. 6d) demonstrated the variety of bacterial adhesions was barely diminished within the PC-Cu MPNs group in comparison with the management (by about twofold), whereas the variety of bacterial adhesions on the PC-Cu@K6-PEG group was diminished by about 100 folds in comparison with the management group and by about 50 folds in comparison with the PC-Cu MPNs. The outcomes have been attributed to the robust hydrophilicity of PEG in PC-Cu@K6-PEG, which shaped a tenacious hydration layer that would strongly repel micro organism after they approached the floor for adhesion [52]. Moreover, Tanaka et al. [53] proposed the “intermediate water idea” to elucidate the water-mediated repulsion. This idea posits that the bodily barrier of intermediate water performs a pivotal position in stopping the adsorption of proteins onto the substrate [50]. The dense intermediate hydration layer shaped by the excessive density of PEG chains on the floor of the coatings efficiently prevented the adhesion of micro organism and proteins on the floor. Collectively, these outcomes reveal that the surface-modified PC-Cu@K6-PEG coatings are extremely immune to the adhesion of proteins and micro organism.
(a) Laser confocal imaging of live-dead-stained S.aureus and E.coli colonized on clean substrates, PC-Cu MPNs coating and PC-Cu@K6-PEG coating; (b) fluorescence semi-quantitative outcomes for them; (c) The colonization conduct of Staphylococcus aureus and Escherichia coli on clean substrates, PC-Cu MPNs coatings, and PC-Cu@K6-PEG coatings; (d) the counting of their numbers have been noticed by SEM. The info was statistically derived from the variety of micro organism adhering to the floor of the pattern (n = 3). The size bars in (a) have been 5 μm and in (c) have been 2 μm. (N = 3, no significance famous as “ns”, *p < 0.05, **p < 0.01, ***p < 0.001 utilizing t-test.)
Hemolysis and antiplatelet adhesion
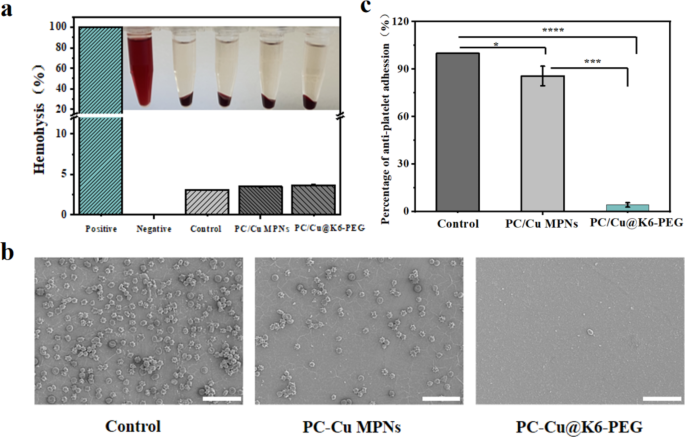
One of the crucial essential standards for the profitable utility of blood-contacting supplies and gadgets is their blood compatibility. That is primarily assessed via in vitro analyses that study the adhesion of blood cells and the formation of blood clots [54,55,56]. The Triton X-100 and 0.9% NaCl answer have been employed because the optimistic and detrimental controls, respectively. Following incubation with erythrocytes at 37 °C for 4 h (Fig. 7a), the hemolysis price was discovered to be lower than 5% for each the PC-Cu MPNs coating and the PC-Cu@K6-PEG coating. This means that the coatings have a low hemolytic potential. It’s effectively established that platelet adhesion to the floor of biomaterials can set off blood coagulation and thrombosis [51, 57], The diploma of platelet deformation and the quantity of platelet adhesion are generally employed as indicators of hemocompatibility in biomaterials [58, 59]. To evaluate the resistance of samples to platelet adhesion, platelet adhesion checks have been carried out utilizing SEM to look at their morphology in addition to quantitative evaluation by counting the variety of adherent platelets. As proven in Fig. 7b, there was a major distinction within the diploma of platelet adhesion on the floor of the completely different samples, with A lot of platelets adhered to the clean silicon substrate (management) (roughly 30,000/cm2), and a comparatively small variety of platelets (roughly 10,000/cm2) adhered to the PC-Cu MPN floor. In distinction, the presence of K6-PEG enabled the coating to successfully inhibit platelet adhesion and aggregation with a price of lower than 50 /cm2, and the discount of platelet adhesion was about 99.84%. Normalised to the variety of platelets adhering to the floor of the management samples in Fig. 7b, as proven in Fig. 7c, we are able to see that the variety of platelets adhering to the floor of the PC-Cu MPNs coatings differs from that of the management group by just one star, whereas the PC-Cu@K6-PEG group reveals a considerably smaller distinction than the latter group. These knowledge recommend PC-Cu@K6-PEG coating exhibited appreciable antithrombotic potential.
(a) Hemolysis take a look at of pink blood cells of clean silicon substrate, PC-Cu MPNs and PC-Cu@K6-PEG coatings; (b) SEM pictures of platelets adhering to reveal and modified polyurethane. (c) Platelet adhesion price normalised to the variety of platelets within the management group in determine b. The size bars in (b) have been 20 μm. (N = 3, no significance famous as “ns”, *p < 0.05, **p < 0.01, ***p < 0.001 utilizing t-test.)
As well as, in an effort to illustrate extra the efficiency of PEG brushes with excessive graft density in anti-biofouling, the outcomes of this examine have been in contrast with these of different research (Desk S2). It may be seen that within the GT’s technique, the best graft density PEG brushes achieved 98.93% anti-sorption of bovine serum proteins and 99% anti-adhesion of platelets. Within the technique of GF, the PEG brush with the best graft density achieved 90% antisorption of human serum proteins and greater than 99.5% antiadhesion to platelets. Within the current examine, antisorption of lysozyme, bovine serum proteins, fibrinogen, mucin, and hemoglobin have been studied, with a most antisorption impact of 99.7% and an antimutagenic impact of greater than 99.8% on platelets (see Desk S2 for extra comparisons).
Ex vivo antithrombogenic properties
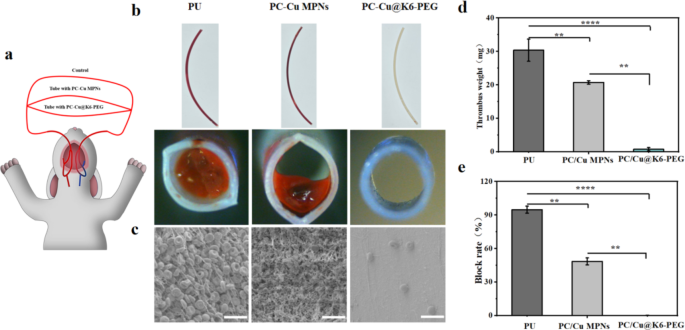
As a way to additional simulate scientific functions, in vitro catheter blood circulation experiments have been performed [60]. Rabbit arteriovenous shunt circuits have been linked utilizing commercially out there medical polyurethane catheters with completely different floor remedies. (Fig. 8a) Following 2 h of ex vivo circulate circulation with none systemic heparin anticoagulation, thrombus formation was discovered to be barely diminished within the PC-Cu MPNs group as compared with the catheter with out coatings (Fig. 8b). Nearly no apparent thrombus formation was noticed within the PC-Cu@K6-PEG group. SEM evaluation moreover demonstrated that the grafting of K6-PEG successfully inhibited thrombus formation (Fig. 8c). The blood-contact surfaces of naked PU and PU-MPNs tubes confirmed extreme thrombosis with properties of densely interconnected polymerized fibrin networks, pink blood cells, and activated platelets, within the PU-PC-Cu@K6-PEG group, solely a really small variety of haemocytes have been seen on the floor (Fig. 8c). Quantitative evaluation of those outcomes additional confirmed that thrombosis was considerably diminished within the PC-Cu@K6-PEG group in comparison with the opposite teams, as evidenced by the evaluation of thrombus weight, occlusion price (Fig. 8d-e). Particularly, PC-Cu@K6-PEG confirmed an roughly 30 folds and 21 folds discount in imply clot weight in contrast with the PU tubing group and the PC-Cu MPNs group, respectively (Fig. 8d) Moreover, the catheter occlusion price was quantified by calculating the proportion of lumen space occlusion utilizing computerised picture evaluation (Fig. 8e). The PC-Cu@K6-PEG modified catheters exhibited a major discount in thrombus occlusion price in comparison with the PU tube group and PC-Cu MPNs group, with an occlusion price of roughly 5%. These outcomes clearly demonstrated that the K6-PEG modification imparts glorious anti-thrombotic properties to the fabric floor.
(a) Schematic illustration of arteriovenous (AV) shunt mannequin linked to the rabbit; (b) Pictures of aspect (high) and cross-section (backside) view of the naked and modified PU tubes after blood circulation; (c) Lumen floor morphology of every pattern after blood circulation characterised by SEM; (d) thrombus high quality; (e) catheter masking ratio.The size bars in (c) have been 20 μm. (N = 3, no significance famous as “ns”, *p < 0.05, **p < 0.01, ***p < 0.001 utilizing t-test.)
The 2 primary components concerned in thrombosis on blood contact implants are fibrinogen (Fib) adsorption and activation and platelet adhesion and activation [61]. Preliminary experiments confirmed the anti-absorptive capability of the K6-PEG-modified floor in opposition to a variety of proteins (together with Fib, hemoglobia, and so on.), in addition to its resistance to platelet adhesion, suggesting a possible anti-thrombotic capability. This was subsequently confirmed by the outcomes of in vitro blood circulation experiments in rabbits. Due to this fact, the PC-Cu@K6-PEG coating has good prospects to be used in medical antithrombotic catheters.