The worldwide annual most cancers mortality has lately reached roughly 10 million, with projections indicating an increase to 22 million by 2035 [1]. The standard strategies for medical most cancers therapy primarily embrace surgical procedure, chemotherapy, and radiotherapy. Surgical procedure can instantly take away apparent stable tumors, however lacks precision in fully eradicating residual most cancers cells or tissues. Furthermore, some sufferers are bodily weak and unable to endure surgical intervention. Chemotherapy has systemic therapeutic results, but in addition has systemic toxicity and important unintended effects [2]. For superior most cancers, 50–70% of sufferers endure radiotherapy. Radionuclide remedy makes use of ionized atoms and free radicals launched by radionuclides to cleave single stranded DNA, thereby inducing most cancers cell apoptosis [3]. As well as, radionuclides can be utilized for single photon emission computed tomography (SPECT) [4] and positron emission tomography (PET) [5, 6], that are the primary strategies for diagnosing tumors and metastases.
Radionuclide remedy faces the next challenges: sure kinds of most cancers are insensitive to RT, non-specific distribution hinders bioavailability, and ionizing injury to non-target organs. The best way to unravel the above issues is to mix radionuclides with small molecule probes and nano-carriers [7,8,9]. Firstly, numerous biomaterials resembling antibodies, peptides, liposomes, nanoparticles, have been developed for radionuclides focused supply [10,11,12]. By utilizing nanoparticles to ship radionuclides particularly to the tumor tissues, the bioavailability of radionuclides is elevated and toxicity to regular tissues is decreased. By adjusting the suitable measurement and floor modification nanoparticles with focused ligand, it has been proven to lengthen blood circulation time and improve the buildup and retention of radionuclides in tumor tissues [13]. Secondly, the tumor microenvironment (TME) is totally different from regular tissues, resembling hypoxia, acidity, and elevated ranges of reactive oxygen species (ROS) [14]. These abnormalities promote tumor invasiveness, metastasis, recurrence, and resistance to therapies together with RT [15]. The nanoparticles design can regulate the TME and enhance RT efficacy. Lastly, high-Z ingredient nanoparticles can be utilized as radiosensitizers to boost RT, particularly treasured steel nanoparticles, which improve the radiation absorption cross-section, enhance the relative dose accumulation of tumors, promote free radical technology, improve DNA injury means, and enhance RT efficacy [16].
Just lately, superparamagnetic iron oxide nanoparticles (SPIONs), silica gold nanoshells, carbon nanoparticles, in addition to radiopharmaceuticals labelled with radionuclides, 64Cu labelled liposomes carrying doxorubicin (Dox) and 89Zr labelled polymeric nanoparticles carrying docetaxel have been used for medical imaging research [17]. The primary medical PET imaging research of 64Cu labelled liposomes carrying Dox was performed in sufferers with main and metastatic breast most cancers. There’s a correlation between the deposition of 64Cu and Dox in tumors and affected person’s illness development free survival interval [18]. In one other vital medical case, 89Zr labelled polymeric nanoparticles carrying docetaxel was utilized in a medical research, and it was discovered that the dimensions of lesion with the best nanoparticle uptake decreased by 50% after 96 h of therapy [19].
Along with imaging, the medical therapeutic utility of 188Re labelled liposomes has additionally been reported [20]. Therapeutic results have been noticed in some metastatic lesions, and SPECT and CT imaging additionally confirmed uptake of 188Re labelled liposomes. Nevertheless, dosimetric research have proven that the liver and spleen of sufferers obtain the best doses, resulting in the termination of medical work. At current, there have been no medical research on radionuclides labelled nanoparticles for mixture remedy. Nevertheless, these preliminary research have obtained regulatory approval for medical use [21, 22], highlighting the potential of nanoparticles together remedy for tumors.
Single remedy is proscribed by tumor resistance, and new drug growth typically encounters such resistance. Nanoparticles cannot solely obtain focused supply of radionuclides to tumor websites, but in addition be utilized in mixture with different therapies resembling PTT and CMT [23, 24]. Mixture remedy entails the usage of two or extra strategies to realize higher therapeutic results, which is predicted to scale back drug resistance, most cancers metastasis, and adversarial reactions. The mix of radionuclides with PTT [8], PDT [10, 25,26,27,28,29], CMT [30], immunotherapy [31], and different therapy strategies to boost the effectivity of most cancers therapy.
This assessment briefly introduces therapeutic radionuclides, summarizes the mixture remedy of RT with PTT, PDT, CMT, immunotherapy, consultant analysis on nanoparticles labelled with radionuclides, and gives a complete overview of their purposes in most cancers therapy. This text critiques the newest progress within the mixture remedy of radionuclides primarily based on multifunctional nanoparticles and different therapy methods, and explores medical research on tips on how to enhance the mixture of RT and different therapies. I hope this assessment can present some steerage for the optimization of radionuclides labelled nanoparticles and corresponding therapy methods.
Radionuclides for remedy
131I radionuclide remedy of thyroid most cancers and hyperthyroidism has a historical past of over 70 years. Bone-targeted radionuclide remedy will be achieved utilizing 223Ra-chloride [32], 89Sr-chloride, 153Sm-[ethylenediamine tetramethylene phosphonic acid] and 188Re-[hydroxyethylene diphosphonate] [33]. Radionuclide remedy of metastatic castration-resistant prostate most cancers contains 177Lu-Prostate particular membrane antigen (PSMA) [34], 225Ac-PSMA [35], 223Ra-dichloride [36] and 188Re-Hydroxyethylidene Diphosphate [37]. The pioneer of Peptide Receptor Radionuclide Remedy (PRRT) is 111In diethylenetriaminepentaacetic acid-D-[Phe1]-Octreotide. 111In emits Auger and conversion electrons, which have potential cytotoxic results [8]. Furthermore, 90Y-DOTA-Tyr3-Octreotide [38] and 177Lu-DOTA0-Tyr3 Octreotate [39] are generally used for PRRT.
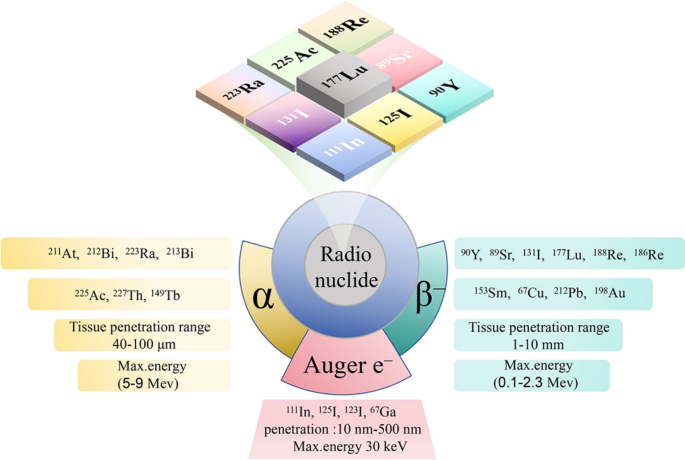
The bodily traits of radionuclide embrace decay modes, emission properties and half-life, whereas their organic traits embrace stability and toxicity in vivo. Furthermore, important biomedical elements embrace the kind, measurement, density, and heterogeneity of focused tumor. Determine 1 and Desk 1 summarizes alpha particle (α), beta particle (β), and Auger electron (AE) decay traits, together with half-life, emission energies, and their penetration ranges inside tissues.
Alpha-particle radiation
Alpha radioactive isotopes possess decrease emission energies (5–9 MeV) and smaller penetration capabilities (40–100 μm) [25]. Notably, the attribute of α radioactive isotopes is excessive linear vitality switch. There was extra intensive injury in particular absorption websites, growing DNA double-strand breaks. Some great benefits of α-emitting nuclides stem from their greater radiobiological effectivity, being much less influenced by tissue oxygenation, dose charges, and mobile resistance, whereas additionally demonstrating decrease toxicity to non-target tissues [40, 41].
Beta-particle radiation
Beta radioactive isotopes possess larger emission energies (0.1–2.3 MeV) and penetration (1–10 mm), enabling the extra uniform deposition of radiation dose to non-target cells by radioactive isotopes sure to adjoining goal cells, even in circumstances of non-uniform distribution on the goal website. Nevertheless, this motion might result in radiation publicity to radiation-sensitive non-target tissues. Furthermore, the cytotoxic impact of β radiation considerably depends on tumor microenvironment, notably cell metabolism and oxygen stage. It’s also doable for sure tumor cells to develop tumor resistance [8].
Auger electron radiation
The vitality of Auger electron is usually lower than 30 keV, with a extremely restricted vary of motion, usually inside 10 nm to 500 nm [42]. Auger electrons launched by radionuclides sometimes solely grow to be efficient upon coming into into the cell nucleus. Metabolites carrying Auger electrons selectively and domestically irradiate the cell membrane and DNA, inducing cell demise [7].
Photothermal remedy
Photothermal remedy represents a non-invasive therapeutic strategy, using near-infrared mild (NIR) to irradiate tumor areas with minimal affect on regular tissue [43]. Tumor cells exhibit weaker tolerance to excessive temperatures in comparison with regular cells, and temperatures of round 45 °C can induce apoptosis [44, 45]. Mixture therapies of radionuclides and photothermal remedy embrace polydopamine (PDA), Pd nanosheets, Au nanoparticles. Besides PTT, Magnetic hyperthermia (MHT) with magnetic iron oxide nanoparticles additionally displays notable therapeutic efficacy (Desk 2).
Polydopamine
Polydopamine has inherent biocompatibility, easy preparation, sturdy near-infrared absorption, excessive photothermal conversion effectivity, and simple floor modification. As a multifunctional materials, PDA has been extensively utilized in biomedical fields [46]. Liu et al. [47] encapsulated single-walled carbon nanotubes with a polydopamine shell, modified with poly(ethyleneglycol) (SWNT@PDA-PEG). SWNTs exhibit sturdy NIR absorbance. 131I used to be labelled to SWNT@PDA-PEG for imaging and radionuclide remedy. Each magnetic resonance imaging (MRI) and gamma imaging demonstrated efficient SWNT@PDA-131I-PEG tumor accumulation. In comparison with monotherapies, the mixed strategy of NIR-triggered PTT and 131I radionuclide remedy resulted in tumor eradication with a outstanding synergistic anti-tumor impact. Anaplastic thyroid most cancers is among the most aggressive malignancies in people, and tumor cells are proof against 131I uptake. Wang et al. [48] synthesized mesoporous PDA nanoparticles with a brain-like channel construction. PDA not solely function 131I nanocarrier with giant particular floor space, but in addition operate as photothermal conversion brokers for PTT. PDA + NIR quickly heated the tumor tissue inside 6 h post-injection, reaching 50 °C inside 5 min, far surpassing management group. Impressively, on account of the mixture of PTT and RT, animal fashions handled with PDA-131I NPs exhibited a formidable therapeutic response. Li and associates [49] developed PDA and surface-functionalized gold nanoparticles (PDA-131I-AuNFs-PEG). SPECT confirmed that 131I-AuNFs exhibited excessive photothermal conversion effectivity, secure radioactivity and successfully accumulating in vivo. Beneath 1064 nm lasers, PTT mixed with RT, the therapy end result far surpassed that of both single remedy. Injection of AuNFs and PDA-131I-AuNFs-PEG into tumor-bearing mice resulted in 57.8% and 100% suppression of tumor progress, respectively.
Along with PDA, researchers have additionally studied different natural nanoparticles labelled with radionuclides. Meng [50] synthesized 131I-labelled Human serum albumin-Indocyaninegreen (131I-HSA-ICG) nanoparticles ranging in measurement from 25 to 45 nm. These nanoparticles exhibited a photothermal conversion effectivity of as much as 24.25% underneath NIR laser. Fluorescent imaging and SPECT confirmed that 131I-HSA-ICG nanoparticles might persist in tumor tissues for 4–6 days. Beneath 808 nm laser, 131I-HSA-ICG demonstrated important ablation on tumor. Zhu and associates [51] developed a novel biocompatible melanin-based nanoprobe (PMNs-II-813) coupled with extremely particular small-molecule inhibitors of prostate-specific membrane antigen, designed for exact multimodal analysis and therapy of prostate most cancers. The Melanin exhibited photoacoustic imaging and photothermal remedy capabilities underneath NIR laser. 89Zr, 131I and Mn2+ have been stably conjugated to the nanoparticles, guiding RT and PTT underneath PET/MRI multimodal imaging. The mix of PTT and RT suppressed prostate most cancers progress (roughly 93% tumor suppression price 20 days post-treatment), considerably outperforming single remedy.
Au
Au nanoparticles possess outstanding qualities like excessive inertness, wonderful biocompatibility, ease of chemical modification, and environment friendly switch capabilities. It has garnered important consideration and undergone intensive analysis within the area of photothermal remedy [13]. Quite a lot of Au nanostructures with various dimensions and shapes, spherical nanoparticles, cubic nanostructures, nanorods, and hole nanoshells, have been exactly synthesized underneath managed situations. Jeon [52] developed 124I labelled gold nanoparticles (124I-Au) as a therapeutic nanoplatform. 124I-Au exhibited efficient photothermal conversion each in vitro and vivo, effectively absorbed by macrophages with out cytotoxicity. Macrophages with 124I-Au have been administered to colon tumors, revealing sturdy indicators on the tumor website. Following NIR laser excitation, the temperature elevated to above 43 °C, leading to a big tumor discount in mice handled with 124I-Au + NIR laser. Mixture remedy can considerably improve therapeutic efficacy towards nonspecific concentrating on of anticancer medication. Zyuzin et al. [53] utilized a nanocarrier (gold nanorods and paclitaxel) labelled with therapeutic radionuclide 188Re (Fig. 2). PTT mixed with RT and chemotherapy exhibited considerably improved effectivity in comparison with monotherapy. Peptide Receptor Radionuclide Remedy (PRRT) depends on the binding of peptides with α and β-emitting radionuclides, requiring excessive doses which can result in potential toxicity. Kjaer [54] investigated 177Lu-DOTA-TATE together with gold nanoshells for PTT in treating small cell lung tumors expressing somatostatin receptors. PTT, counting on the warmth induced by gold nanoparticles, complemented PRRT. In comparison with PRRT alone, mice handled with gold nanoshells PTT mixture remedy 1 day after injection of 177Lu-DOTA-TATE confirmed suppressed tumor progress and longer survival time.
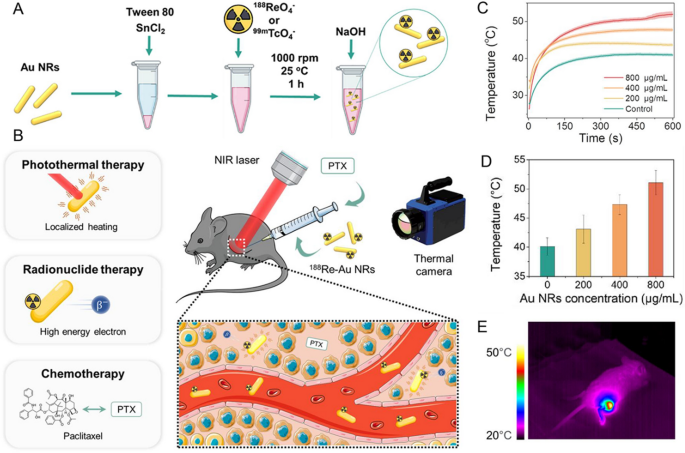
Supply: Improvement of Nanocarrier-Based mostly Radionuclide and Photothermal Remedy in Mixture with Chemotherapy in Melanoma Most cancers Remedy
A, B Mixture remedy utilizing 188Re/99mTc-labelled Au NRs and Paclitaxel (PTX). C Temperature evolution curves of Au NRs at totally different concentrations. D Common temperature reached after 600 s of NIR laser irradiation of tumors with injected Au NRs at various concentrations. E Thermal photos of a mouse with intratumorally injected 800 μg/mL Au NRs irradiated with 1064 nm NIR laser. Copyright {2023} American Chemical Society.
Pd nanosheets
Pd nanosheets, as a photothermal agent, has good biocompatibility, excessive photothermal conversion effectivity, and urine clearance price. Via structural engineering, resembling ultra-thin Pd nanosheets, their photothermal impact will be enhanced, and NIR absorption will be improved [55]. Zheng and associates [56] have reported a pH-sensitive multifunctional therapeutic platform, 131I and 125I labelled Pd nanosheets. 131I-Pd-PEG is comparatively secure in acidic or barely acidic options however unstable in impartial and barely alkaline options, making it a really perfect tumor microenvironment-sensitive therapeutic nanoplatform. Improved therapeutic results have been noticed in mice with suppressing tumor progress upon injection of 131I-Pd-PEG. In one other research performed by the identical group [57], fluorinated palladium nanosheet labelled with numerous radionuclides was used for guiding mixture remedy. Fluorinated Diethylenetriaminepentaacetic dianhydride-PEG-SH (FDP-Pds) was labelled with 124I, 125I, 131I, 177Lu, 99mTc, and 64Cu. Beneath laser excitation, the temperature on the tumor website quickly elevated from 37 °C to 50 °C in 7 min, demonstrating wonderful photothermal remedy. 125I/131I-labelled probes for RT exhibited excellent therapeutic results. The therapeutic results of 131I-FDP-Pds + laser group markedly surpassed these of the remaining teams, with no noticed recurrence throughout a 2-month follow-up interval.
Superparamagnetic iron oxide nanoparticles
Magnetic hyperthermia makes use of magnetic nanoparticles to generate warmth underneath an exterior alternating magnetic discipline, uniformly heating tumors, and has been studied to beat RT resistance [58]. Stanković [59] performed analysis on 131I-CC49 antibodies, which have been conjugated to superparamagnetic iron oxide nanoparticles functionalized with 3-aminopropyltriethoxysilane (APTES). This conjugation exhibited distinctive heating effectivity, with a selected absorption price of 15.9 kA∙m−1. After utility to LS174T human colon carcinoma xenografts, it supplied particular and sustained native retention. The mix of magnetic hyperthermia and RT resulted in tumor quantity suppression charges of 73.00%, considerably inhibiting tumor progress. Bilewicz [60] reported a core–shell nanoparticle (SPIONs coated with a layer of 198Au). This nanoparticle exhibited a excessive saturation magnetization, able to reaching 43 °C at a magnetic discipline frequency of 386 kHz. The vitro experiment outcomes indicated a considerably cytotoxic impact. At a radioactive focus of two.5 MBq/mL, cell survival charges had dropped to under 8% after 72 h. Furthermore, this group [61] employed 225Ac-modified magnetite nanoparticles, which has no compromise to its magnetic properties. The 225Ac@Fe3O4-3-phosphonopropanoic acid-trastuzumab bioconjugate, covalently linked by way of phosphonopropanoic acid, particularly focused ovarian and breast most cancers cells. This bioconjugate displayed excessive cytotoxicity towards SKOV-3 ovarian most cancers cells. Its excessive particular uptake permits it to realize higher therapeutic ends in the mixture of RT and magnetic hyperthermia. Combining therapies represents a promising technique in most cancers therapy. PEI-Mn0.5Zn0.5Fe2O4 nanoparticles (PEI-MZF-NPs) functioned as each the magnetic medium for magnetic fluid hyperthermia and as gene switch vectors for gene remedy. Lin [62] developed PEI-MZF-NPs with anti-afpmcab monoclonal antibody for concentrating on. The suspension uncovered to a magnetic discipline quickly elevated in temperature, maintained at round 43 °C, considerably inhibiting the proliferation of liver tumor cells, far surpassing chemotherapy with doxorubicin. The mix remedy of RT and magnetic hyperthermia demonstrated considerably superior therapeutic results in comparison with single remedy.
Photodynamic remedy
Photodynamic remedy stands as a non-invasive therapeutic strategy, providing a comparably decrease incidence of unintended effects than conventional therapies. Beneath photoexcitation, the photosensitizer (PS) transitions from the bottom state to excited state after which to longer-lived, lower-energy triplet excited state. In Kind I PDT, the photosensitizer reacts with organic substrates to type ROS, resembling hydroxyl radicals, hydrogen peroxide, and superoxide anions. In Kind II PDT, the triplet excited state of the photosensitizer instantly transfers vitality to O2, changing it to singlet oxygen (1O2) [63]. The lifetime of ROS ranges from 0.03 to 0.18 ms, thus exerting toxicity solely to cells in shut proximity to the photosensitizer. There’s an pressing want for a brand new technique to handle the constraints of typical nanoparticle-based PDT, resembling tissue penetration constraints, reliance on exterior mild sources, and restricted accumulation of photosensitizers.
Photosensitizers excited by Cherenkov radiation (CR) can break the constraints of exterior mild penetration. Found in 1934 by Soviet physicist Pavel Alekseevich Cerenkov [64], Cherenkov radiation happen native polarization alongside their trajectory, when charged particles transferring quicker than the pace of sunshine in a medium. The medium releases saved vitality as seen photons, which leads to the attribute blue mild of Cherenkov radiation. The radionuclides can induce photosensitizers to generate ROS by Cherenkov radiation vitality switch [65, 66]. The depth of radiation is instantly associated to the particle’s vitality, enabling exact administration of therapeutic radiation doses to the tumor website. Furthermore, the detection of CR will be utilized for real-time monitoring of PDT, contributing to the optimization of subsequent therapy plans. These advantages underscore the potential of CR-PDT as a safe and environment friendly strategy to most cancers remedy (seek advice from Desk 3).
Porphyrin
Porphyrin and its derivatives are generally used for PDT, and display inherent tumor-targeting properties, which positions porphyrin derivatives paired with appropriate therapeutic radionuclides as potential candidates for focused remedy. Deng [67] developed 188Re-labelled tetra-phenyl porphyrin (TPPS4). Equally, Luo et al. [68] ready 188Re-labelled tetra-[3,4-bis(carboxymethyleneoxy)phenyl] porphyrin. Each analysis teams studied traits resembling yield, particular exercise, in vitro stability, affinity, and biodistribution. 188Re-TPPS4 exhibited excessive tumor affinity and sustained accumulation traits in tumors, however its therapeutic efficacy was not evaluated. Venakatesh [69] synthesized a water-soluble 177Lu-5,10,15,20-tetra[4-carboxymethoxyphenyl]porphyrin, with a selected exercise of 550 TBq/g and a radioactive isotope purity of 99.98%. It displayed 99% radiochemical purity and stability. In a preliminary efficacy research on a mouse mannequin fibrosarcoma tumor, tumor progress was considerably suppressed. As for focused PDT, Watanabe [70] designed a easy in vivo pharmacokinetic analysis system. The gastrin-releasing peptide receptor was used as tumor-targeting peptide. Conjugate of Protoporphyrin IX (PPIX) and the bombesin (BBN) was synthesized as focused agent with 64Cu labeling. [64Cu]PPIX-PEG6-BBN exhibited excessive uptake in PC-3 cells. As a consequence of PPIX’s lipophilicity, it quickly accrued within the liver and kidneys, had a protracted circulation time within the blood and decrease distribution within the tumor. Khalaj [71] ready 166Ho-labelled 5,10,15,20-tetra(3,4-dimethoxyphenyl) porphyrin (TDMPP) and 5,10,15,20-tetra(3,4,5-trimethoxyphenyl)porphyrin (TTMPP), each exhibiting favorable radiochemical purity and particular exercise. These two compounds demonstrated much less hepatic accumulation, thus warranting consideration for his or her potential therapeutic properties. Luo et al. [72] ready 131I-labelled 5,10,15,20-tetrakis (4-hydroxyphenyl) porphyrin (TPPOH) and 5,10,15,20-tetrakis (4-aminophenyl) porphyrin (TPPNH2) for each RT and PDT. Upon 400–600 nm photoexcitation, the tumors handled with 131I-TPPOH and 131I-TPPNH2 exhibited a discount in tumor quantity of 64 ± 11% and 54 ± 10%, respectively, 14 days post-treatment.
Cherry et al. [73] utilized Y-90 as Cherenkov radiation to induce porphyrin-based photosensitizer tetrasulfonated alkylated metalloporphyrin (TPPS2a). TPPS2a exerted not less than 20% therapeutic efficacy towards C6 glioma cells throughout the vary of 6–60 µCi/nicely. The present concentrating on effectivity of radionuclide-labelled nanoparticles is sort of low, thereby limiting the effectivity of CR-PDT. Cai et al. [74] developed 89Zr-labelled deferoxamine-porphyrin nanocomplex (89Zr-Df-PPN). Excessive doses of Df-PPN have been used as CR vitality receivers for passive tumor concentrating on, whereas low doses of 89Zr-labelled Df-PPN acted as CR vitality donors. Based mostly on homology, Df-PPN and 89Zr-Df-PPN have been co-localized to the utmost extent on the tumor website, CR-PDT technique reaching appreciable injury to tumor blood vessels and inhibited tumor progress considerably. Optimized temporal and spatial settings maximized the efficacy of focused CR-PDT and concurrently decreased off-target results. This group [75] additionally investigated a size-controllable dual-tumor-mitochondria-targeting porphyrin-PEG (PPN). Efficient uptake of 177Lu-PPN by tumors and mitochondria considerably enhanced the efficacy of RT and PDT. Tumor progress was fully suppressed after 14 days with 177Lu-PPNs-10 nm underneath laser 660 nm. In one other research, this group [76] synthesized magnetic nanoparticles (MNPs) with floor modification with 89Zr and porphyrin (89Zr-MNPs/TCPP) for CR-PDT and magnetic concentrating on tumor. Tumor accumulation enhanced by exterior magnetic fields. The tumor progress of mice handled with 89Zr-MNPs/TCPP was considerably suppressed.
The technique of using mixture remedy involving two or extra therapies goals to beat tumor resistance skilled with single therapies. Cai et al. [77] assembled copper sulfide nanoparticles on the outside of 89Zr-labelled hole mesoporous silica nanoshells full of porphyrin molecules, aiming to imaging and remedy. The synergistic interplay between copper-mediated PTT and porphyrin-mediated PDT resulted in full tumor eradication inside a day, with no obvious relapse or unintended effects. Moreover, this strategy achieves simultaneous quadmodal (Positron emission tomography/Cerenkov luminescence/Cerenkov radiation vitality switch/Fluorescence) imaging, enabling the applying of multimodal image-guided remedy. Qiu et al. [78] mixed tetraphenylporphyrin (a photosensitizer) and hapten (a chemotherapeutic drug) utilizing cleavable sulfone linkers to supply a ROS-responsive agent. Beneath 68Ga Cerenkov radiation excitation, porphyrin generated ROS, which subsequently cleaved the sulfur-based linkers, resulting in inner agent launch. 68Ga-NOTA-Nb109 as an intracellular CR emitter, this ROS-responsive agent exhibited considerably greater toxicity to A375-hPD-L1 cells, owing to the mixture of PDT and CMT.
Titanium dioxide
Titanium dioxide (TiO2) nanoparticles are thought-about to be biocompatible and cost-effective therapeutic useful nanomaterials. TiO2 nanoparticles have been confirmed efficient as photosensitizers for most cancers therapy. The bandgap vitality of TiO2 is 3.2 eV. Beneath CR excitation, the bottom state of TiO2 nanoparticles transitions to excited state and generates ROS. Hilefu [79] reported that 18F-FDG induced an O2-independent photosensitizer, transferrin-coated TiO2 nanoparticles (TiO2–Tf). Inside three days, tumor quantity notably decreased by 40 ± 5%. Non-invasively monitoring the distribution of TiO2 NPs within the physique stays elusive. This group [80] loaded Gd onto transferrin-coated TiO2 NPs. TiO2-Gd-Tf maintained ROS technology functionality at vast concentrations and induced important cell demise. Gd distinction brokers facilitated MRI monitoring of NPs distribution and accumulation inside tumors. Liu et al. [81] in contrast 68Ga and 18F, demonstrating that 68Ga is a more practical CR-PDT radionuclide. The Cherenkov effectivity of 18F is comparatively low, requiring dosages 10–30 occasions greater than traditional to realize therapeutic results. 68Ga as CR supply to induce D-glucose-modified TiO2, 68Ga-labelled bovine serum albumin (68Ga-BSA) exhibited suppression of 4T1 cell progress in comparison with 18F labelled 2-fluoro-deoxyglucose (18F-FDG). Achilefu [82] developed a therapeutic nanocomposite, TiO2 coated with tumor-targeting transferrin and 89Zr (Fig. 3A). 89Zr-TiO2-Tf generated ROS, triggering apoptosis and finally decreasing the tumor quantity by tenfold. AcPark [83]developed an CR-induced 89Zr-TiO2-MnO2 nanocomposite, which binds transferrin to the nanoparticle floor, inhibiting most cancers cell progress. MnO2 transformed tumor space acidic H2O2 into O2, thereby enhancing the efficacy of CR-PDT. Overexpressed glutathione in tumor cells will be depleted by MnO2, disrupting the antioxidant protection system and selling PDT (Fig. 3B, C). Furthermore, inherent MnO2 can take up Cherenkov radiation, facilitating ROS technology and inhibiting tumor progress. Totally different system parameters (nanoparticle measurement, nanoparticle focus, and radioactivity of radionuclides) can affect the amount of Cherenkov photons and ROS technology. Biswas [84] developed a mathematical mannequin that integrates Cherenkov physics, light-matter interactions and photocatalytic response. This mannequin described the pathway of CR-induced 18F-FDG/TiO2 to generate ROS. The generated ROS elevated with the rise of TiO2 focus. The focus of ROS first elevated with the rise of particle measurement, reached a peak at round 800 nm, after which decreased. This was primarily because of the dominance of photoexcitation and e−/h+ technology when the particle measurement is under 800 nm.
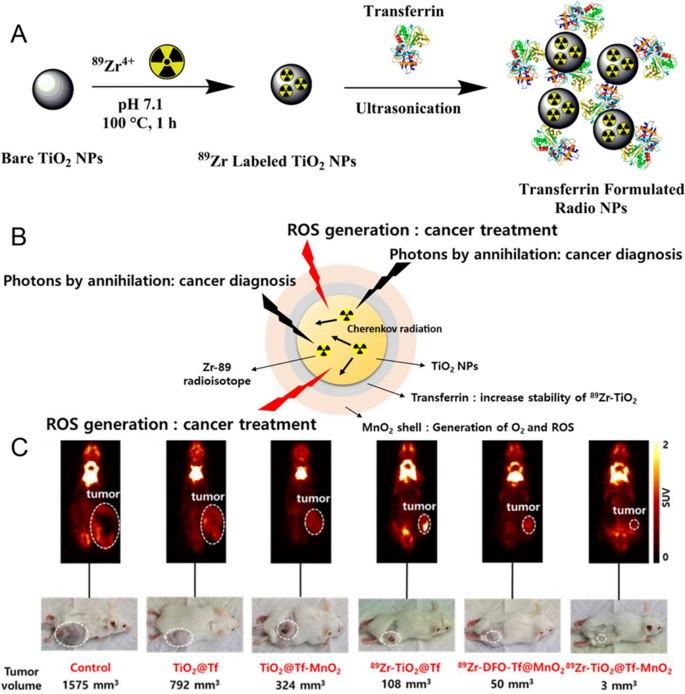
Supply: Osteotropic Radiolabeled Nanophotosensitizer for Imaging and Treating A number of Myelom, Theranostics by way of Using Cherenkov Radiation of Radioisotope Zr-89 with a Nanocomposite Mixture of TiO2 and MnO2
A Schematic illustration of 89Zr-labelled of TiO2 NP and 89Zr-TiO2-Tf. Copyright {2020} American Chemical Society. B Kind of theranostic 89Zr − TiO2@Tf-MnO2. C.18F-FDG PET photos and tumor pictures after CR-PDT. Copyright {2023} American Chemical Society.
Different photosensitizers
The analysis on photosensitizers for PDT primarily focuses on natural nanoparticle porphyrins and inorganic nanoparticle TiO2. Different kinds of photosensitizers have additionally been studied together remedy. Gao et al. [85] developed 131I-labelled 5-aminolevulinic acid onto exosome-mimetic vesicles (131I-EM@ALA). Apart from radionuclide remedy, 131I used to be additionally used to CR mild supply. Via the mixture of RT and CR-PDT, 131I-EM@ALA induced DNA injury and activated the lysosomal-mitochondrial pathway to kill tumor cells. Compared to the management group (0%, 24 days), the 131I-EM@ALA group confirmed considerably extended survival charges (12.5%, 35 days). Within the context of disseminated a number of myeloma cells, Achilefu et al. [86] reported a high-selective supply and co-localization nanocomposite composed of HSA, transferrin, titanocene and 18F-FDG. The CR-induced nanocomposite demonstrated suppression of tumor progress in extremely metastatic breast most cancers fashions. Downregulation of dimeric protein targets CD49d resulted in enhanced therapeutic resistance in small cluster carcinoma cells, thereby increasing the applying to beforehand untreatable metastatic illnesses.
Injecting radionuclides and photosensitizers individually don’t assure their efficient interplay within the tumor website. A typical strategy to handle this concern is thru the nanoparticles self-assembly. Solar [87] developed a self-assembly 131I-labelled photosensitizer (131I-sPS), which composed of porphyrin-a (photosensitizer), diisopropyl (pH-sensitive moiety), and 131I-labelled tyrosine (CR provider). As a consequence of aggregation-induced quenching, 131I-sPS exhibited decrease photo-toxicity in regular tissues, however might generate ROS on the tumor website upon decomposition. Following intravenous injection, it demonstrated wonderful tumor inhibition in subcutaneous 4T1-tumor mice. Miao [88] developed a glycol chitosan-based nanoparticles (GCP-NPs) consisting of pyropheophorbide-a. GCP-NPs exhibited pH-dependent floor cost conversion, and switched its floor cost from damaging to optimistic in acidic tumor microenvironment, enhancing mobile internalization and penetration, bettering the tumor accumulation. Furthermore, 99mTc of SPECT imaging in a subcutaneous mouse tumor mannequin confirmed an 8.1-fold enhance in tumor uptake in comparison with GCP-NPs. In contrast with different teams, 177Lu-GCP-NPs mixed RT with PDT, demonstrated a really perfect tumor progress suppression price of 95.5% (Fig. 4).
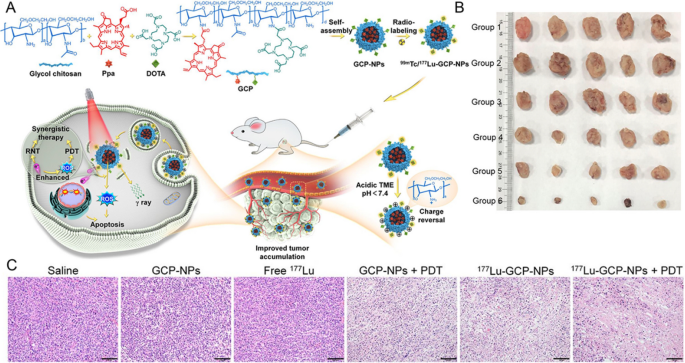
Supply: Acidity-Activated Cost Conversion of 177Lu-Labeled Nanoagent for the Enhanced Photodynamic Radionuclide Remedy of Most cancers
A Schematic illustration of 99mTc/177Lu-GCP-NPs and the mechanism of mixture remedy. B Pictures of tumors after therapies. C Histological H&E staining of tumors after therapies. Scale bar: 100 μm. Copyright {2022} American Chemical Society.
Cai et al. [89]synthesized hole mesoporous silica nanoparticles (HMSN) loading Ce6 and 89Zr. Ce6 may very well be induced by 89Zr CR. Vivo research revealed that subcutaneous injection of 89ZrHMSN-Ce6 had a suppressive impact on tumor progress, leading to a 75% discount in tumor quantity. Zhang [90] developed ultra-small nanoclusters, derived from NH2-Ti32O16. The introduction of dopamine (DA) ligands not solely promoted the water solubility and photocatalytic efficiency, but in addition engaged in tumor-targeting. Beneath 18F-FDG CR excitation, it generated hydroxyl radicals, the place holes (h+) reacted with H2O to carry out kind I PDT, and the transferred e− achieved Ti3+ enhancement, considerably bettering chemotherapy (Fig. 5). Radionuclides and CR can be utilized as inner mild sources to induce photosensitizers for PDT of deep tumors. Nevertheless, the low effectivity of CR limits its therapeutic impact. Solar et al. [91] developed a 131I-labelled zinc tetra(4-carboxyphenoxy) phthalocyaninate, which conjugated Cr3+ doped zinc gallate nanoplatform (131I-ZGCs-ZnPcC4) for RT and CR-PDT. 131I might induce ZGCs by Cherenkov luminescence and ionizing radiation, permitting it to sustainably emit mild and induce ZnPcC4 photosensitizer for PDT. 131I-ZGCs-ZnPcC4 demonstrated wonderful tumor suppression each in vitro and vivo. Together remedy of RT and PDT, PDT is generally induced by CR. Together with radionuclides 131I, 89Zr, 18F, and so forth. CR radiation vitality is low, and a few researchers have launched high-energy exterior mild sources. Rijpkema [92] synthesized high-affinity and extremely tumor-targeting 111In-labelled nanocomposite composed of PSMA ligands, fluorophore, and IRDye700DX. In comparison with white mild, nanocomposite underneath 690 nm excitation for fluorescence-guided surgical procedure resulted in decreased tumor recurrence and considerably extended survival.
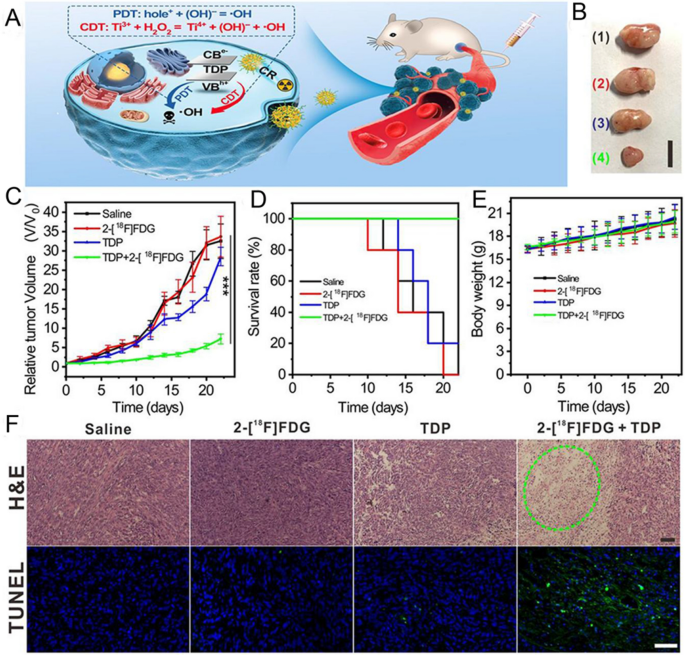
Supply: Ligand Engineering of Titanium-Oxo Nanoclusters for Cerenkov Radiation-Strengthened Picture/Chemodynamic Tumor Remedy
A Schematic illustration of mixture remedy. B Dimension of tumor on the twenty second day publish therapy in every group from as much as under: saline, 2-[18F] FDG, TDP, and TDP + 2-[18F] FDG (scale bar: 1 cm). C Common tumor progress curves after therapies. D Survival curves after therapies. E Weight progress curves after therapies. F Consultant photos of the tumor dissection stained by H&E and TUNEL after therapies. (Scale bar: 50 μm). Copyright {2021} American Chemical Society.
Chemotherapy
The idea of mixture remedy originates from chemotherapy, integrating the localized or systemic administration of varied radiolabeled-molecules, chemotherapeutic brokers, or different compounds. This mix of RT and CMT provides a extra customized strategy to most cancers therapy. Nanoscale platform, resembling liposome and micelle, permits for the encapsulation of a number of therapeutic compounds and radionuclides. Furthermore, concentrating on modification with antibodies, antibodies fragments, and peptides facilitate the amalgamation of varied therapies. Chemotherapeutic brokers studied together with RT embrace doxorubicin, paclitaxel and alkylating-agents et al. (Desk 4).
Doxorubicin
Doxorubicin is a extensively used anticancer anthracycline antibiotic that accumulates within the nucleus. Shieh [93] loaded Dox onto multifunctional micelles. Tumors handled with 188Re-Dox micelles decreased mobile proliferation and exhibited extra necrotic options. Liu [94] found 131I-99mTc-PDA-PEG/Dox nanoparticles for the mixture of tumor focused radiotherapy and chemotherapy. The murine tumor mannequin exhibited important effectivity in most cancers therapy in comparison with single remedy. Furthermore, no important toxicity was noticed in handled mice inside 80 days. Ningthoujam [95] developed 177Lu-labelled NaGdF4/Ho-Yb up-conversion nanoparticles, coated with a biocompatible m-SiO2 layer and Dox. Beneath 980 nm excitation, up-conversion nanoparticles emit pink and inexperienced mild. Following the motion of NaGdF4/Ho-Yb@mSiO2-Dox, enhanced elimination of most cancers cells was noticed. Pilip [96] synthesized 213Bi-labelled cubic liquid crystalline phases with the Dox and amphiphilic ligand, which resulted in a big lower in survival functionality of HeLa most cancers cells.
Auger electrons are very low-energy electrons emitted by radionuclides. This vitality is deposited inside a really brief vary (< 0.5 µm), leading to excessive linear vitality switch and deadly injury to most cancers cells. Subsequently, AEs have nice potential in most cancers therapy. Ogawa [97] developed a 125I-labelled Dox liposome for AEs remedy. The liposome accrued extremely in most cancers tissue, upon warmth induction, launched 125I-labelled Dox by-product into the goal website. 125I-Dox by-product accrued in nucleus, leading to excessive most cancers cell toxicity by emitting AEs. Thisgaard [98] ready AEs-emitting 125I-5-iodo-2-deoxyuridine (125I-UdR) together with thymidine synthase inhibitors, methotrexate, and temozolomide. The mix remedy considerably decreased the viability of glioblastoma cells in distinction to 125I-UdR alone.
Paclitaxel and camptothecin
Paclitaxel (PTX) has been accredited by the Meals and Drug Administration as a radiation sensitizing drug. Chilkoti [99] investigated the synergistic results of 131I-labelled thermoresponsive elastin-like polypeptide and PTX nanoparticles. Nanoparticles conjugated with albumin and paclitaxel, efficiently surmounted drug resistance, demonstrating therapeutic effectivity towards tumors in each subcutaneous and in situ pancreatic tumor fashions. Denkova [100] reported polymer micelles with PTX and 177Lu, combining CMT with RT, demonstrating higher cell progress inhibition and cytotoxic results. Xi [101] developed a 131I-labelled hybrid hydrogel nanoparticle, self-assembled with carboxymethyl cellulose (CMC), bovine serum albumin, and camptothecin for mixture remedy. It exhibited a considerable drug-loading capability, a low hemolysis price, and pH-dependent drug launch, considerably decreasing injury to regular tissues. Furthermore, it successfully promoted intracellular uptake, extended blood circulation time, and enhanced accumulation inside tumor tissues. The mix remedy had proven wonderful in vivo anti-cancer results, considerably higher than monotherapy.
Immunotherapy
Particular person RT has restricted penetration depth and uneven dose distribution, leading to a “blind spot” of residual stay most cancers cells and growing the chance of tumor metastasis. RT can stimulate anti-tumor immune response by inducing immunogenic cell demise [102, 103]. The combination of radionuclide remedy with immunotherapy (inner radio-immunity remedy, IRIT) goals to perform efficient synergism (Desk 5). Yang and associates [104] developed a 131I-labelled supply platform primarily based on bacterial outer membrane vesicles, coating with tyrosine-rich protein statherin and PEG. It might stimulate dendritic cell maturation and generate anti-tumor cytokines, strengthen the systemic immune reminiscence results induced by radioimmunotherapy and inhibit tumor invasion.
Close to-infrared photoimmunotherapy (NIR-PIT) is an strategy that binds to antibody specificity however is proscribed by low tissue penetration of NIR mild. Kobayashi [105] reported 18F-FDG CR-induce IR700 for PIT. Whereas CR-PIT can induce deep tumor destruction in vivo, its effectivity just isn’t as pronounced as that of NIR-PIT because of the shorter-wavelength mild. Furthermore, the efficacy of each RT and immunotherapy is hindered by the tumor microenvironment and immunosuppression. Liu et al. [106] developed ZnFe(CN)5NO monolayer nanosheets and proposed the usage of in-situ chemiluminescence to launch NO for tumor microenvironment modulation. Upon 32P labeling, CR induced ZnNO (32P) nanosheets to launch NO, which not solely inhibited HIF-1a expression, alleviated tumor hypoxia, and enhanced the efficacy of RT, but in addition regulated the immunosuppressive tumor microenvironment. Combining anti-programmed cell demise protein 1 with ZnNO (32P) nanosheets might activate distant results in immune checkpoint blockade remedy, inhibiting the expansion of distant metastatic tumors.
Bu and associates [107] reported 131I-labelled Au nanoparticles and double-arginine translocation peptide (131I-AuNPs-TAT). 131I supplied excessive focus of beta radiation to probably the most radiation-sensitive DNA. Concurrently, when the rate of beta particles decreased, electrons rushed to the AuNPs, resulting in the conversion of low-dose X-rays. X-rays have a larger depth of penetration than beta-rays and might induce a robust immune response, thereby decreasing the unseen areas by short-range beta radiation. Combining RT with immunotherapy not solely enhances the efficacy of RT but in addition broadens the 131I medical purposes. In comparison with management group, the tumor progress of was considerably suppressed within the 131I-AuNPs group and the 131I-AuNPs-TAT group. Yang [108] synthesized 99mTc/177Lu-labelled glutathione-modified Au nanoclusters. RT induced immunogenic cell demise, activating immune cells, regulating the expression of tumor cell PD-L1. This indicated that anti-PD-L1 blockade has important synergistic results in inhibiting distant tumors, offering long-term immune reminiscence safety, and considerably bettering the standard of life and lifespan of transgenic mice. Regardless of Au nanoparticles being an inert steel that ought to not disrupt regular physiological capabilities, research [109] have discovered that Au nanoparticles can stimulate immune responses and affect therapy outcomes. AuNPs have been proven to change gene expression related to cleansing, lipid metabolism, and hepatic protection responses. Bigger AuNPs (50 nm) quickly elevated the expression of IL-1β, IL-6, and TNF-α in organs. Nevertheless, smaller AuNPs (10 nm) solely led to a milder hepatic cytokine response.
Chemodynamic remedy (CDT) makes use of Fenton or Fenton-like reactions to control the immunogenic tumor microenvironment. Nevertheless, relying solely on CDT just isn’t ample to activate immune responses for suppressing tumor metastasis and recurrence, a necessity arises for mixture therapies of immunotherapy and RT. Yu et al. [110] designed a 211At-labelled Mn-based radioimmunotherapy promoter. 211At was used to focused alpha remedy. Apart from serving as a provider, MnO2 generated •OH by way of CDT and enhanced immune checkpoint blockade (ICB), inhibiting the first tumor with out recurrence.