Sustained excessive ranges of oxidative stress in diabetic wound tissue
Present analysis signifies that in regular wounds, oxidative stress primarily happens inside the preliminary three days following damage and step by step diminishes because the inflammatory part subsides [7]. In distinction, diabetic wounds exhibit sustained, excessive ranges of ROS [3]. To additional substantiate the oxidative stress ranges inside diabetic wounds, we have now established a rat mannequin of diabetes mellitus (DM) following the protocol delineated in Fig. 1A. The monitoring of blood glucose within the Ctrl group and the DM group confirmed the profitable institution of the diabetes mannequin (Fig. 1B). Subsequently, a full-thickness incision with a diameter of 20 mm was made on the dorsal floor of each the Ctrl group and the DM group rats. As illustrated in Fig. 1C, the postoperative wound therapeutic technique of each rat teams was documented at days 0, 1, 3, 5, 7, 14, and 21. In comparison with Ctrl, the DM group exhibited a noticeably delayed wound therapeutic course of with an inclination in direction of non-closure (Fig. 1D). Contemplating the pivotal position of oxidative stress in wound therapeutic, we examined the tissue oxidative stress ranges at numerous phases of wound therapeutic (Day 3 – the inflammatory part, Day 7 – the proliferation part, and Day 14 – the transforming part [1]) in each the Ctrl and DM teams. The outcomes point out that, whether or not on the third, seventh, and 14th day of wound therapeutic, the DM group exhibited considerably decrease actions of superoxide dismutase (SOD) and protein expression of heme oxygenase-1 (HO-1) in comparison with the Ctrl group. Furthermore, the degrees of lipid peroxidation (malondialdehyde, MDA) and expression of cyclooxygenase-2 (COX2) had been notably larger within the DM group than within the Ctrl group (Fig. 1E-G). The aforementioned experimental outcomes point out that, compared to regular wounds, diabetic wound tissues constantly preserve excessive ranges of oxidative stress all through your entire wound therapeutic course of, corroborating earlier analysis findings.
Institution of the Diabetic Wound Mannequin and detection of oxidative stress ranges in tissues. (A) Schematic illustration of the institution of the diabetic wound mannequin. (B) Blood glucose ranges within the management group (Ctrl) and the diabetic group (DM). (C) Consultant images of diabetic wounds for Ctrl and DM teams on the 0, 1st, third, fifth, seventh, 14th, and twenty first day. (D) Quantitative evaluation of wound space change compared with the unique wound. (E) SOD and MDA stage of Ctrl and DM on day 3, 7 and 14. (F) Corresponding immunohistochemistry staining of COX2 and HO-1 on day 3, 7 and 14. (G) Immunohistochemical quantification of COX2 and HO-1 in picture F (n = 5). *P < 0.05, **P < 0.01, ***P < 0.005
Excessive-throughput screening of pure product libraries and community pharmacology evaluation
To higher goal oxidative stress and enhance the therapeutic impact on diabetic wounds, this research used TBHP (100 µM) to induce oxidative stress in HUVECs in vitro, and a high-throughput screening was carried out on the pure product library (NPL) consisting of 622 compounds (Determine S1A). The particular process is illustrated in Fig. 2A, the place cell viability is employed as an indicator of a drug’s capability for antioxidant stress. Toxicity exams had been carried out on the highest 5 rating medication primarily based on cell viability to determine the optimum dosages for these 5 medication (Determine S1B). On the optimum focus, the fluorescence depth of DCFH-DA was utilized to evaluate the ROS scavenging capabilities of those 5 compounds (Fig. 2B). Subsequently, baicalein (BAI, 5 µM), which exhibited probably the most outstanding antioxidative impact in HUVECs, was chosen for additional investigations.
Utilizing community pharmacology, a Venn diagram was constructed to elucidate the anticipated targets of PC and BAI, in addition to the disease-related targets concerned in diabetic wound therapeutic. Moreover, frequent targets shared by these compounds had been recognized (Fig. 2C and S1C). With a purpose to examine the respective results of organic supplies and prescribed drugs on illness remedy, we carried out separate Gene Ontology (GO) Enrichment Evaluation for the frequent targets of BAI and PC that intersect with the illness however don’t overlap. The outcomes of the enrichment evaluation point out that BAI could affect on peptidyl-tyrosine phosphorylation and mobile response to hydrogen peroxide (Fig. 2D), whereas PC primarily impacts the vascular endothelial development issue receptor signaling pathway (Fig. 2E). This means that BAI and PC exhibit a possible complementarity in therapeutic efficacy, and their mixed motion could affect wound therapeutic via distinct processes, particularly antioxidant stress and angiogenesis. This opens up the potential of multifunctional optimization for PPNs.
Excessive-throughput Drug Screening, Community Pharmacology, and GO Enrichment Evaluation. (A) The method diagram and analytical outcomes of high-throughput drug screening in NPL. (B) Results of various medication with TBHP on ROS ranges in HUVECs (n = 3). *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001, vs. that of Baicalein. (C) Venn diagram for the potential goal of procyanidins and baicalein and the illness targets of diabetic wound therapeutic. (D) Bubble chart of the organic course of class phrases from GO enrichment evaluation for the 77 frequent targets. (E) Bubble chart of the organic course of class phrases from GO enrichment evaluation for the 11 frequent targets
Synthesis and characterization of baicalein-procyanidins polyphenol nanovesicles (BPPNs)
PC can make the most of the bodily adsorption of CaCO3 on polyphenols to type vesicles [22, 23], which might de-nucleate into PPNs beneath acidic circumstances. Below the identical circumstances, new polyphenol vesicles, BPPNs had been synthesized utilizing equal quantities of PC and BAI. PPNs and BPPNs are collectively known as “Polyphenol nanovesicles (PNs)”. As proven in Fig. 3A, the scanning electron microscopy (SEM) picture of PPNs and BPPNs reveals that BPPNs are spherical vesicles with a diameter smaller than PPNs. The diameter and Zeta potential of each had been measured, and it was discovered that the diameter of BPPNs was about 500 nm, which is half of PPNs, and the Zeta potential of BPPNs was much like PPNs, indicating that the addition of BAI decreased the dimensions of the vesicles with out affecting their stability (Fig. 3B). The FTIR spectra of BAI, PPNs, and BPPNs are depicted in Fig. 3C. No free hydroxyl peak may be present in PPNs, as indicated by a broad absorption peak at 3310 cm-1, suggesting that hydroxyl hydrogen bonds bind PC molecules related [12]. The BPPNs inherit the phenolic hydroxyl absorption peak uncovered by BAI (3409 cm-1), signifying that, at a chemical stage, the newly synthesized nanoparticles exhibit enhanced antioxidative properties. Moreover, compared to BAI, BPPNs exhibit no absorption peak round 1655 cm-1, implying that the ketone carbonyl disappeared throughout the synthesis technique of the nanovesicles, a doable addition response occurred between the carbonyl group within the BAI construction and the phenolic hydroxyl group in PC. This doubtlessly led to the formation of hemiketals that successfully protect the chemical construction of BAI. Apart from, the addition of BAI leads to a big weakening of the infrared absorption peaks and the disappearance of sure group vibrations, as noticed within the BPPNs’ FTIR spectrum. This means that intermolecular forces are fashioned between BAI and PC. Given the presence of phenolic hydroxyl teams, we will infer that the intermolecular forces at the very least contain hydrogen bonding [12]. The presence of intermolecular forces additionally explains the discount in particle measurement, which is attributed to heterogeneous nucleation [24]. Primarily based on this, create a synthesized illustrative diagram as depicted in Fig. 3D. Analyzing from a chemical perspective, the hemiketals can shield the carbonyl construction in BAI, decompose after pH change, and launch full BAI and PC molecules beneath the mildly acidic setting present in diabetic wounds [25], respectively taking part in their respective therapeutic roles. In accordance with the molecular components (PC: C30H26O13, BAI: C15H10O5) and XPS evaluation of PNs involving carbon (C) and oxygen (O) atoms, the outcomes point out that the content material of PC and BAI in BPPNs is roughly in a 1:1 ratio (Fig. 3E). The height becoming outcomes additionally confirmed the profitable synthesis of BPPNs (Fig. 3F). In abstract, it may be inferred that in BPPNs, PC, and BAI can bear proportional addition response to generate hemiketals, that are then related by intermolecular forces to synthesize smaller-diameter polyphenol nanovesicles.
With a purpose to additional examine the organic exercise of PNs, toxicity exams had been carried out on PNs (Determine S2 A and B). The utmost protected focus for subsequent experiments was decided to be 4 µg/ml (After conversion, the content material of BAI and PC in 4 µg/ml BPPNs is roughly 5 µM every). To facilitate the in vitro monitoring of the extracellular uptake of PNs, we employed the fluorescent dye FITC to label PNs. Below each circumstances—with and with out TBHP stimulation, mobile uptake of PNs was recorded at 1 h and a couple of h (Fig. 3G). Quantitative outcomes, as proven in Fig. 3H, reveal that each PPNs and BPPNs exhibit time-dependent and ROS-responsive uptake. Notably, BPPNs exhibit a larger mobile uptake effectivity in comparison with PPNs, which can be attributed to the smaller measurement and elevated ease of mobile internalization related to BPPNs. After co-treatment with PNs and TBHP for 2 hours, the localization relationship between PNs and mobile organelles was assessed utilizing LysoTracker and MitoTracker (Fig. 3I and J). The quantitative outcomes of Pearson’s correlation coefficient point out that each varieties of vesicles can obtain lysosomal escape and co-localize with mitochondria beneath oxidative stress (Fig. 3Ok).
In abstract, in contrast with PPNs, the newly synthesized BPPNs have been optimized in characterization. BPPNs are a novel kind of polyphenolic nanovesicles with smaller particle measurement, larger mobile uptake effectivity, lysosomal escape, and mitochondrial ROS response properties.
The Synthesis and Characterization of PPNs and BPPNs. (A) SEM picture of PPNs and BPPNs. Scale bar: 500 nm. (B) Particle measurement and Zeta potential of PPNs and BPPNs. (C) FTIR spectra of BAI, PPNs and BPPNs. (D) Schematic of PPNs and BPPNs synthesis. (E) XPS full spectrum of PPNs and BPPNs. (F) The high-resolution XPS spectrum for O1s of BPPNs. (G) Photographs of HUVECs taking over PPNs and BPPNs. Inexperienced: FITC-labeled PNs; Blue: Hoechst (nuclei). Scale bar: 100 μm. (H) Quantitative measurement of FITC fluorescence depth in G. (I) Confocal microscope pictures finding FITC-labeled PPNs and BPPNs relative to lysosomes in HUVECs cells with TBHP. Inexperienced: FITC-labeled PNs; Purple: LysoTracker (lysosome); Blue: Hoechst (nuclei). Scale bar: 10 μm. (J) Confocal microscope pictures finding FITC-labeled PPNs and BPPNs relative to mitochondrions in HUVECs cells with TBHP. Inexperienced: FITC-labeled PNs; Purple: MitoTracker (mitochondrion); Blue: Hoechst (nuclei). Scale bar: 10 μm. Ok. Pearson’s correlation coefficients indicating the diploma of PNs/lysosome and PNs/Mitochondrion colocalization in I and J : R = 1 (good colocalization), R = 0 (no colocalization). n = 3, *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001
In vitro angiogenic exercise of BPPNs
Primarily based on the community pharmacology and enrichment evaluation outcomes from Fig. 2C and E, it’s speculated that PC could take part in or have an effect on the VEGF sign pathway throughout the technique of diabetes wound therapeutic. In response to VEGF stimulation, endothelial cells sprout from the capillaries after which full your entire technique of angiogenesis with the proliferation, migration, and tubular formation of those cells, finally offering vitamins to the wound tissue, which is a key step in wound therapeutic [1, 26].
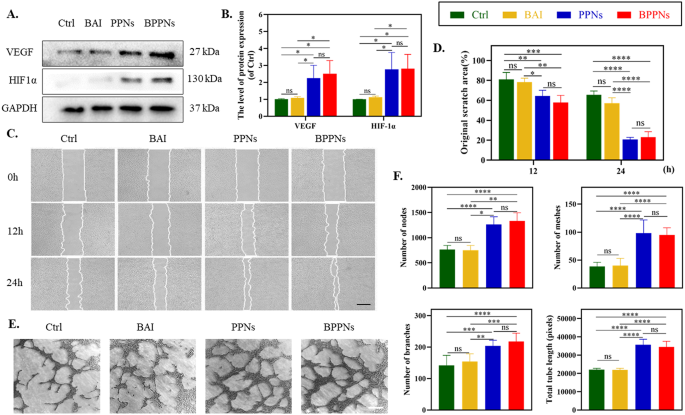
To additional make clear the influence of PNs on angiogenesis, we carried out related exams on HUVECs. Scratch assays had been carried out on hooked up HUVECs. Western blot outcomes indicated that each BPPNs and PPNs successfully upregulated the expression of vascular angiogenesis-related proteins, together with VEGF, and its upstream regulator HIF-1α (Fig. 4A, B). The cells had been handled with BAI (5 µM), PPNs (4 µg/ml), or BPPNs (4 µg/ml) for twenty-four h, with HUVEC migration recorded at 0 h, 12 h, and 24 h (Fig. 4C). The outcomes reveal that, when in comparison with the management group, BAI exhibited no important influence on HUVECs’ migration. Nevertheless, PPNs synthesized by way of PC considerably enhanced scratch therapeutic. Notably, BPPNs containing BAI even have related cell migration-promoting results as PPNs (Fig. 4D). Following this, we carried out tube formation assays on HUVECs from completely different remedy teams on the Matrigel matrix (Fig. 4E), and quantified the variety of nodes, meshes, branches, and complete tube size (Fig. 4F). The outcomes demonstrated that BAI had virtually no impact on the tube formation of HUVECs, whereas BPPNs exhibited a promotion of tube formation exercise much like that of PPNs. General, though BAI doesn’t have an effect on tube formation, BPPNs can successfully upregulate angiogenic-related proteins, promote the migration and tube formation of HUVECs, and procure related selling tube exercise as PPNs.
The angiogenic properties of BPPNs in vitro. A–B. Western blots of VEGF and HIF-1α by HUVECs incubated in several preparations for twenty-four h. C. Consultant pictures of HUVEC migration after treating for 0, 6, 12 h, scale bar: 500 μm. D. Quantify the migration of HUVEC. E. Consultant pictures of HUVEC tubular formation after treating for 4 h, scale bar: 200 μm. F. Quantify the tubular formation of HUVEC. n = 3, *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001
In vitro antioxidant exercise of BPPNs
To additional discover the in vitro antioxidant exercise of BPPNs, HUVECs had been divided into 5 teams as proven in Fig. 5, with a remedy length of 24 h, and a sequence of exams had been carried out. The CCK-8 outcomes indicated that BAI, PPNs, and BPPNs may all mitigate the discount in cell viability brought on by TBHP, with the rescue impact growing within the order of PPNs < BAI < BPPNs (Fig. 5A). In comparison with the Ctrl group, the oxidative stress stage in HUVECs of the TBHP group was considerably elevated (marked by a lower in SOD exercise and a rise in MDA content material), whereas the antioxidative stress capabilities of PPNs, BAI, and BPPNs had been enhanced (Fig. 5B). ROS probes and JC-1 staining had been carried out on HUVECs beneath completely different remedies (Fig. 5C and E). Oxidative stress induced by TBHP led to a rise in intracellular ROS and mitochondrial harm, with BPPNs exhibiting the very best functionality in counteracting ROS and repairing mitochondrial harm, adopted by BAI (Fig. 5D and F). Subsequently, it’s speculated that the distinguished antioxidant exercise of BPPNs is essentially attributed to BAI, which was recognized via high-throughput screening within the NPL.
Primarily based on the outcomes of community pharmacology and enrichment analyses (Fig. 2C and D), it’s hypothesized that the distinctive antioxidative efficacy of BAI could also be attributable to its regulatory operate on tyrosine phosphorylation. Protein phosphorylation modification, significantly prevalent and functionally paramount, happens in over 30% of mobile proteins. Regardless of tyrosine phosphorylation modifications (P-Tyr) constituting lower than 1% of all protein phosphorylation modifications, they play a pivotal position in practically each physiological course of inside the cell [27, 28]. Research have indicated {that a} high-fat food regimen in mice can have an effect on the P-Tyr of liver proteins [29]. Proteins inside pathways equivalent to JAK1/STAT3 [30, 31], Erk1/2 [32], and Akt [33], all containing tyrosine residues, are topic to elevated ranges of tyrosine phosphorylation as a consequence of oxidative stress, thereby activating their respective signaling pathways. To validate the influence of BPPNs on P-Tyr and their antioxidative results on the protein stage, Western blot experiments had been carried out (Fig. 5G). The outcomes revealed that BAI considerably inhibits the tyrosine phosphorylation of pathway proteins JAK1, STAT3, Erk1/2, and Akt, markedly modulates oxidative stress-related proteins (upregulating HO-1 and downregulating COX-2), and suppresses the expression of the mitochondria apoptosis-related protein Cyto C. These modulatory results are notably superior to these of PPNs however inferior to these of BPPNs (Fig. 5H). Determine 5I used to be drawn primarily based on the above experimental outcomes and literature search [34, 35].
In abstract, BPPNs can successfully counteract in vitro oxidative stress brought on by TBHP by inhibiting tyrosine phosphorylation of pathway proteins, whereas additionally taking part in a task in mitochondrial safety (Fig. 5I).
In vitro antioxidant exercise of BPPNs. (A) Results of various preparations with TBHP on cell viability in HUVECs. (B) Results of various preparations on SOD and MDA stage in HUVECs. (C) DCFH-DA staining of HUVECs with completely different preparations. Scale bar: 100 μm. (D) Results of various preparations on ROS ranges in HUVECs. E–F. JC-1 staining pictures of HUVECs with completely different remedies. JC-1 aggregates: regular mitochondria (pink); JC-1 monomers: unhealthy mitochondrial (inexperienced). Scale bar: 100 μm. G–H. Western blots of p-JAK1, JAK1, p-STAT3, STAT3, p-Erk1/2. Erk1/2, p-Akt, Akt, HO-1, COX2 and Cyto C by HUVECs incubated in several remedies. I. The antioxidant mechanism diagram of BPPNs. n = 3, *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001
BPPNs speed up diabetic wound therapeutic
In consideration of the appliance of PNs in diabetic wounds, we employed a photosensitive hydrogel with tissue adhesiveness, hyaluronic acid with o-nitrobenzene (HA-NB), to anchor the PNs on the wound websites [36, 37]. The institution of a diabetic wound mannequin in SD rats adopted by the random allocation of those topics into 4 teams, as depicted in Fig. 6. Initially, we evaluated the influence of remedies with HA-NB, PPNs@HA-NB, and BPPNs@HA-NB on the principal organs—coronary heart, liver, spleen, lungs, and kidneys—via H&E staining of organ tissue sections. The absence of pathological alterations in these stained sections means that HA-NB, PPNs@HA-NB, and BPPNs@HA-NB don’t exhibit organ toxicity, as illustrated in Supplementary Fig. 3.
Determine 6A and B depict the wound therapeutic development over fourteen days throughout 4 remedy teams, accompanied by a quantification of the wound areas (Fig. 6C). In comparison with the DM group, the appliance of HA-NB considerably enhances the therapeutic of diabetic wounds, probably attributable to the decreased threat of an infection on the diabetic wound websites facilitated by the protection supplied by HA-NB [37]. It’s significantly noteworthy that the BPPNs@HA-NB group exhibited the next price of diabetic wound therapeutic in comparison with the PPNs@HA-NB group. Moreover, we employed laser Doppler scan pictures to characterize the purposeful vasculature with blood circulation (Fig. 6D). It was noticed that BPPNs@HA-NB may successfully upregulate angiogenesis across the wound throughout the proliferative part of therapeutic, thereby abbreviating the extended proliferative part induced by diabetes (Fig. 6E). Subsequent histopathological analysis of the neo tissue on day 14 post-injury utilizing H&E and Masson’s trichrome staining is offered in Fig. 6F. Quantitative analyses, as proven in Fig. 6G, revealed that diabetic wound tissues handled with BPPNs@HA-NB had thicker granulation tissue, shorter wound lengths, and better collagen deposition in comparison with the PPNs@HA-NB group. These findings collectively counsel that BPPNs considerably speed up diabetic wound therapeutic and promote the development of wound therapeutic processes in diabetes, compared to PPNs.
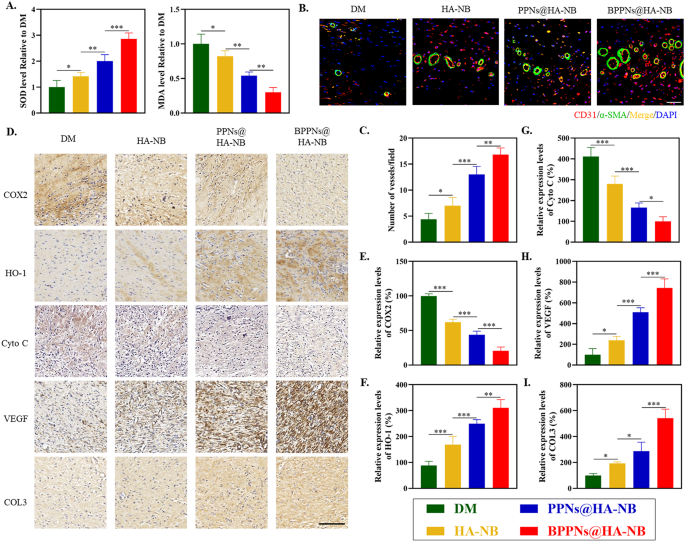
To research the intrinsic mechanisms underlying the therapeutic efficacy of BPPNs in treating of diabetic wounds, we additional assessed the degrees of oxidative stress, angiogenesis, and collagen inside the tissue 14 days post-injury. The detection outcomes for Superoxide Dismutase (SOD) and Malondialdehyde (MDA) indicated that the BPPNs@HA-NB group considerably decreased oxidative stress ranges in diabetic wound tissues in comparison with the opposite three teams (Fig. 7A). Immunofluorescence co-staining for CD31 and α-SMA, markers of neovascularization (Fig. 7B), together with the quantification of fluorescence expression proven in Fig. 7C, demonstrated that the vascular density within the BPPNs@HA-NB group was comparatively larger. As depicted in Fig. 7D, immunohistochemical evaluation of oxidative stress-related markers (COX2 and HO-1), mitochondrial apoptosis markers (Cyto C), angiogenesis-related markers (VEGF), and Collagen III (COL3) revealed that BPPNs@HA-NB may downregulate COX2 and Cyto C protein expression and upregulate the relative expression of HO-1, VEGF, and COL3 in comparison with the opposite three teams. These findings counsel that BPPNs could speed up diabetic wound therapeutic by counteracting oxidative stress, defending mitochondria, selling angiogenesis, and enhancing collagen deposition.
In abstract, the newly synthesized polyphenol vesicles, BPPNs, optimized with BAI, exhibit enhanced efficacy in counteracting the extreme oxidative stress encountered throughout diabetic wound therapeutic in comparison with the unique anthocyanin polyphenol vesicles, PPNs. Moreover, BPPNs retain the angiogenic-promoting results of PPNs whereas their superior antioxidant exercise creates a extra “fertile floor” for angiogenesis. Consequently, the outcomes point out that BPPNs exhibit enhanced in vivo angiogenesis promotion in comparison with PPNs. General, BPPNs speed up diabetic wound therapeutic via a twin strategy: combating oxidative stress and selling angiogenesis, highlighting their potential as a multifaceted therapeutic intervention in diabetic wound administration (Graphical summary).
In vivo analysis of BPPNs within the diabetic rat full-thickness wounds mannequin. (A) Consultant photographic pictures of the injuries therapeutic course of with completely different remedies on day 0, 1, 3, 5, 7 and 14. (B) Traces of wound-bed closure throughout 14 days for every remedy. (C) proportion of wound space after remedy with the completely different preparations on day 1, 3, 5, 7 and 14. (D) Consultant Laser Doppler scan pictures on the diabetic wound after remedy on day 0, 1, 3, 5, 7 and 14. (E) Quantification of blood circulation quantity at day 1, 3, 5, 7 and 14 utilizing moorLDI Evaluate V6.1 software program. (F) H&E and masson staining analysis of wound regeneration after remedy with the completely different preparations on the 14th day. The black dashed line present the border between the wound tissue and surrounding wholesome pores and skin tissue. (G) Quantification of granulation tissue thicknesses, the size of wound and collagen quantity fraction for various remedies on the 14th day. n = 5, *P < 0.05, **P < 0.01, ***P < 0.005
Analysis of the therapeutic efficacy after BPPNs remedy. (A) Results of various preparations on SOD and MDA stage on day 14. (B) Immunofluorescence staining of neovascularizationat the wound tissue with completely different remedies on day 14. α-SMA (inexperienced), CD31 (pink), and DAPI (blue). Scale bar: 100 μm. (C) Quantitative evaluation of the blood vessel density on day 14. (D) Corresponding immunohistochemistry staining of COX2, HO-1, Cyto C, VEGF and COL3 on day 14. Scale bar: 200 μm. E–I. Immunohistochemical quantification of COX2, HO-1, HIF-1α, VEGF and COL3, respectively. n = 5, *P < 0.05, **P < 0.01, ***P < 0.005